Question
Question: Which of the following can exist as a pair of enantiomers:...
Which of the following can exist as a pair of enantiomers:
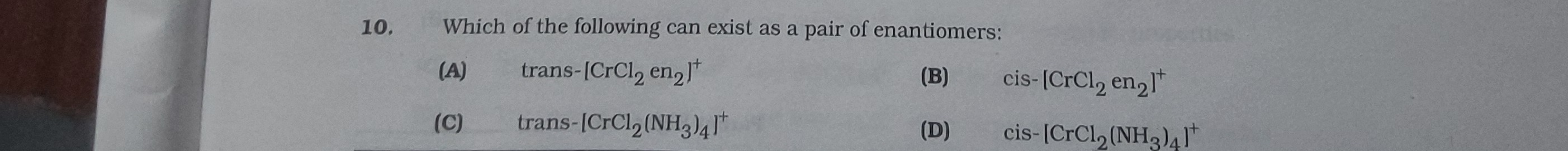
trans-[CrCl2 en2]+
cis- [CrCl2 en2]+
trans-[CrCl2(NH3)4]+
cis-[CrCl2(NH3)4]+
B
Solution
To determine which of the given complexes can exist as a pair of enantiomers, we need to identify the chiral complexes. A chiral molecule is non-superimposable on its mirror image and lacks any improper axis of rotation (Sn), including a plane of symmetry (σ, which is S1) and a center of inversion (i, which is S2). All the given complexes are octahedral.
Let's analyze each option:
(A) trans-[CrCl2 en2]+
This complex is of the type trans-[M(AA)_2 X_2], where M = Cr, AA = en (ethylenediamine, a bidentate ligand), and X = Cl.
In the trans isomer, the two Cl ligands are positioned 180 degrees apart. If we place the Cr atom at the origin, and the two Cl ligands along the z-axis, then the two bidentate en ligands lie in the equatorial (xy) plane.
This geometry possesses several symmetry elements:
-
A plane of symmetry passing through the Cr atom and the two Cl ligands, and bisecting the C-C bonds of the two
enligands. -
A plane of symmetry passing through the Cr atom and the four nitrogen atoms of the
enligands (the equatorial plane). -
A center of inversion at the Cr atom.
Due to the presence of these symmetry elements, the trans-[CrCl$_2$ en$_2$]$^+$ complex is achiral and cannot exist as a pair of enantiomers.
(B) cis-[CrCl2 en2]+
This complex is of the type cis-[M(AA)_2 X_2].
In the cis isomer, the two Cl ligands are positioned 90 degrees apart (adjacent).
This cis isomer lacks a plane of symmetry and a center of inversion. It is, therefore, chiral.
A molecule and its mirror image for cis-[M(AA)_2 X_2] are non-superimposable, meaning they are enantiomers. This is a classic example of optical isomerism in coordination compounds.
(C) trans-[CrCl2(NH3)4]+
This complex is of the type trans-[MA_4 X_2], where M = Cr, A = NH3 (monodentate ligand), and X = Cl.
In the trans isomer, the two Cl ligands are 180 degrees apart. The four NH3 ligands lie in the equatorial plane.
This complex has multiple planes of symmetry:
-
A plane of symmetry passing through the Cr atom and the four NH3 ligands (the equatorial plane).
-
A plane of symmetry passing through the Cr atom and the two Cl ligands, and two of the NH3 ligands.
-
A center of inversion at the Cr atom.
Due to these symmetry elements, trans-[CrCl$_2$(NH$_3$)$_4$]$^+$ is achiral and cannot exist as a pair of enantiomers.
(D) cis-[CrCl2(NH3)4]+
This complex is of the type cis-[MA_4 X_2].
In the cis isomer, the two Cl ligands are 90 degrees apart. The four NH3 ligands occupy the remaining positions.
This complex possesses a plane of symmetry. Imagine the two Cl ligands and two of the NH3 ligands lying in a plane. This plane acts as a mirror plane, reflecting the other two NH3 ligands onto each other.
For example, if the two Cl ligands are on the y and z axes, and two NH3 ligands are on the x axis, then the yz-plane (containing Cr, the two Cl, and two NH3) is a plane of symmetry, reflecting the two NH3 ligands on the x-axis.
Therefore, cis-[CrCl$_2$(NH$_3$)$_4$]$^+$ is achiral and cannot exist as a pair of enantiomers.
Based on the analysis, only cis-[CrCl$_2$ en$_2$]$^+$ is chiral and can exist as a pair of enantiomers.
Explanation of the solution:
Chirality in coordination compounds requires the absence of a plane of symmetry and a center of inversion.
-
trans-[CrCl2 en2]+: Possesses a plane of symmetry (equatorial plane containing Cr and four N atoms of en ligands) and a center of inversion. Achiral.
-
cis-[CrCl2 en2]+: Lacks a plane of symmetry and a center of inversion. Chiral, exists as enantiomers.
-
trans-[CrCl2(NH3)4]+: Possesses a plane of symmetry (equatorial plane containing Cr and four NH3 ligands) and a center of inversion. Achiral.
-
cis-[CrCl2(NH3)4]+: Possesses a plane of symmetry containing Cr, the two Cl ligands, and two NH3 ligands. Achiral.
Thus, only cis-[CrCl2 en2]+ can exist as a pair of enantiomers.
