Question
Question: Select the incorrect order of atomic radii....
Select the incorrect order of atomic radii.
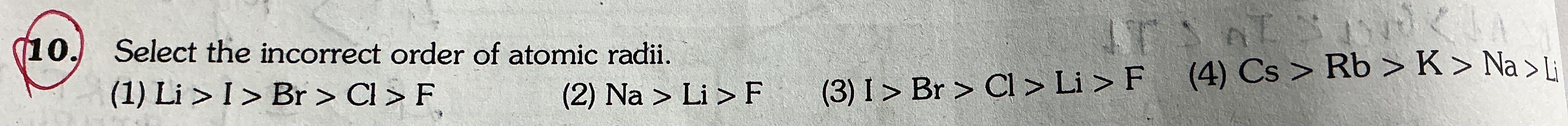
A
Li > I > Br > Cl > F
B
Na > Li > F
C
I > Br > Cl > F
D
Cs > Rb > K > Na > Li
Answer
(1)
Explanation
Solution
Atomic radius increases down a group and decreases across a period. In option (1), Li (Period 2) is compared with I, Br, and Cl (Periods 5, 4, and 3, respectively). Elements in higher periods have larger radii. Thus, I, Br, and Cl are larger than Li, making the order Li > I > Br > Cl > F incorrect.
