Question
Question: 10 Most stable carbocation among the following is :...
10 Most stable carbocation among the following is :
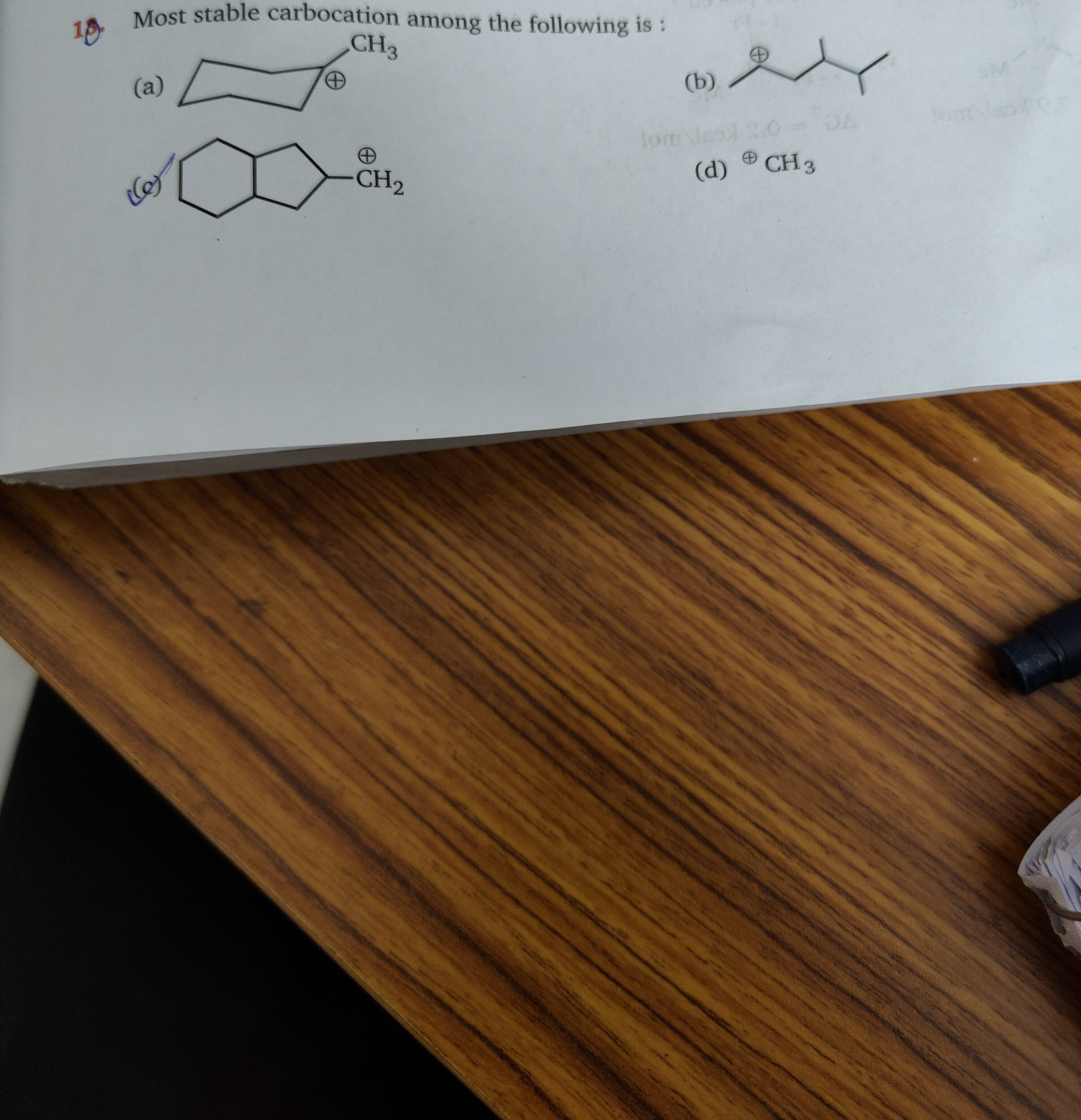
CH3+
(b)
Solution
The stability of carbocations is influenced by inductive effects, resonance, and hyperconjugation. The general order of stability is Tertiary > Secondary > Primary.
-
Option (d) CH3+: This is a methyl carbocation, which is a primary carbocation with no hyperconjugation. It is generally the least stable.
-
Option (c) Primary carbocation: This depicts a primary carbocation within a fused bicyclic system. While primary carbocations are typically unstable, rigid fused ring systems can sometimes offer unique stabilizing effects through sigma bond delocalization or strain relief, though direct resonance is absent.
-
Option (a) Secondary carbocation: This is a secondary carbocation within a cyclohexane ring. It has alpha hydrogens and is stabilized by hyperconjugation and inductive effects from adjacent carbons.
-
Option (b) Secondary carbocation: This is a secondary carbocation CH3−CH+−CH(CH3)−CH2−CH3. The carbocation center is adjacent to a tertiary carbon. This arrangement provides significant stabilization through both hyperconjugation (from the 3 alpha hydrogens on the methyl group and 1 alpha hydrogen on the adjacent tertiary carbon) and a strong inductive effect from the electron-donating tertiary carbon.
Comparing the options:
- Primary carbocations (c and d) are less stable than secondary carbocations (a and b).
- Between the secondary carbocations (a) and (b), option (b) is more stable due to the presence of an adjacent tertiary carbon, which enhances inductive stabilization and provides more alpha hydrogens for hyperconjugation compared to option (a).
Therefore, option (b) is the most stable carbocation among the given choices.
