Question
Question: Value of x in the above reaction is:...
Value of x in the above reaction is:
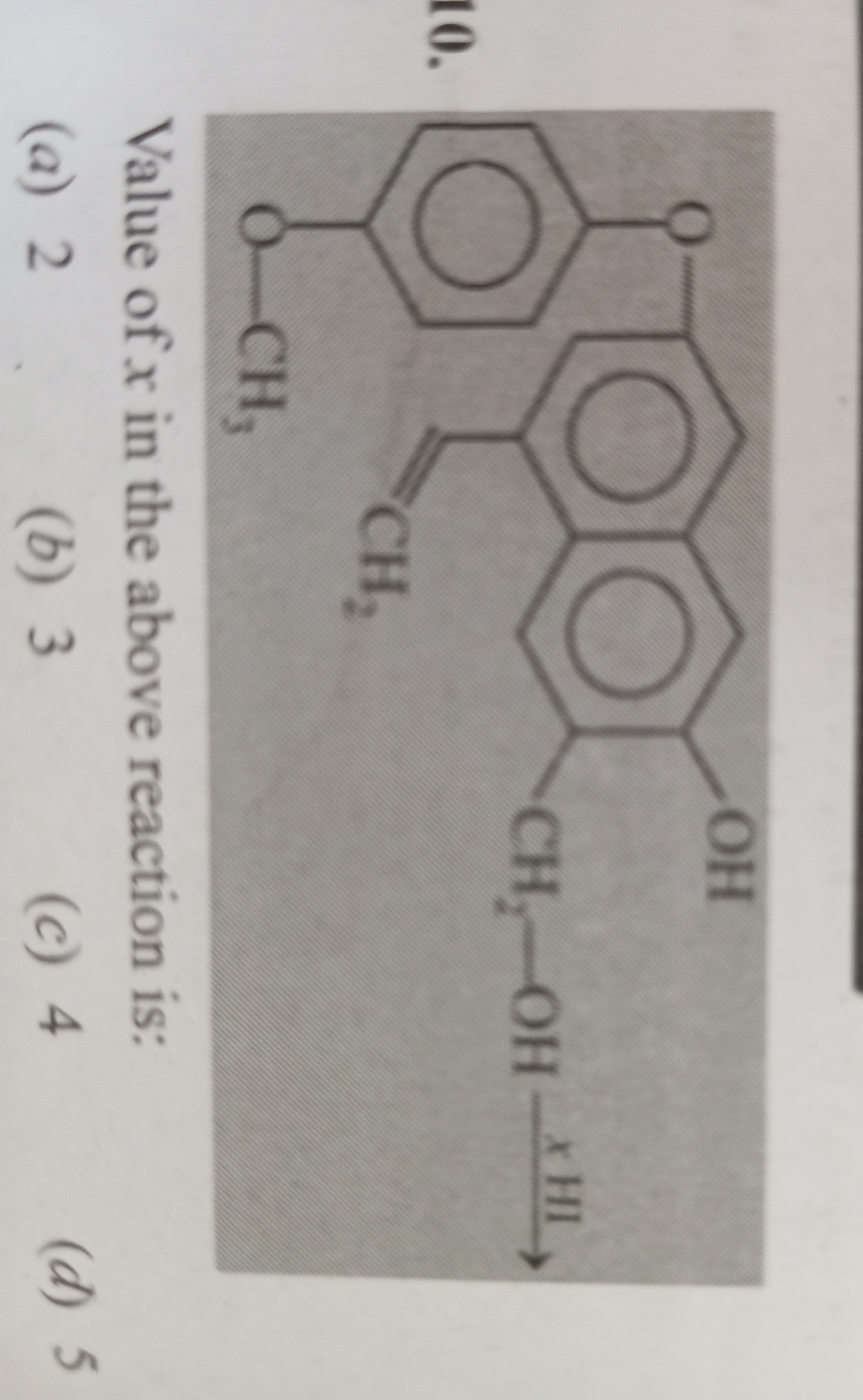
2
3
4
5
3
Solution
The given reaction involves an organic molecule reacting with 'x' moles of HI. To find the value of 'x', we need to identify all the functional groups in the reactant molecule that react with HI and determine the stoichiometry of each reaction.
The reactant molecule is a substituted naphthalene derivative. Let's identify the functional groups:
-
Aryl alkyl ether (-O-CH3): The methoxy group (-OCH3) is attached to one of the benzene rings of the naphthalene system. Aryl alkyl ethers undergo cleavage with HI to form a phenol and an alkyl iodide.
Ar-O-R+HI⟶Ar-OH+R-IIn this case, the methoxy group will be cleaved to form a phenolic hydroxyl group and methyl iodide (CH3I). This reaction consumes 1 mole of HI.
-
Primary alcohol (-CH2-OH): The hydroxymethyl group (-CH2-OH) is a primary alcohol. Alcohols react with HI to form alkyl iodides and water.
R-OH+HI⟶R-I+H2OThus, the primary alcohol group will be converted to an alkyl iodide. This reaction consumes 1 mole of HI.
-
Alkene (-CH=CH2): The vinyl group (-CH=CH2) is an alkene. Alkenes undergo hydrohalogenation (electrophilic addition) with HI. According to Markovnikov's rule, the hydrogen adds to the carbon with more hydrogen atoms, and the iodine adds to the carbon with fewer hydrogen atoms.
R-CH=CH2+HI⟶R-CH(I)-CH3This addition reaction consumes 1 mole of HI.
-
Phenolic hydroxyl group (-OH): There is a hydroxyl group directly attached to one of the benzene rings of the naphthalene system. This is a phenolic hydroxyl group. Phenols generally do not react with HI to form aryl iodides under typical reaction conditions. The carbon-oxygen bond in phenols has partial double bond character due to resonance with the aromatic ring, making it very strong and resistant to nucleophilic substitution by iodide ions.
Counting the moles of HI consumed by each reactive functional group:
- Ether cleavage: 1 mole HI
- Alcohol substitution: 1 mole HI
- Alkene addition: 1 mole HI
- Phenolic -OH: 0 moles HI
Total moles of HI consumed (x) = 1 + 1 + 1 = 3.
Thus, the value of x in the reaction is 3.
