Question
Question: 10. $CF_3I + NaOH \longrightarrow A+B$ $CH_3I + NaOH \longrightarrow P+Q$ (i) pcc (ii) $CH_3MgBr$ ...
- CF3I+NaOH⟶A+B
CH3I+NaOH⟶P+Q (i) pcc
(ii) CH3MgBr
(iii) H3O⊕
(iv) B
C+D (sodium salt of poisonous acid)
Find the correct statement.
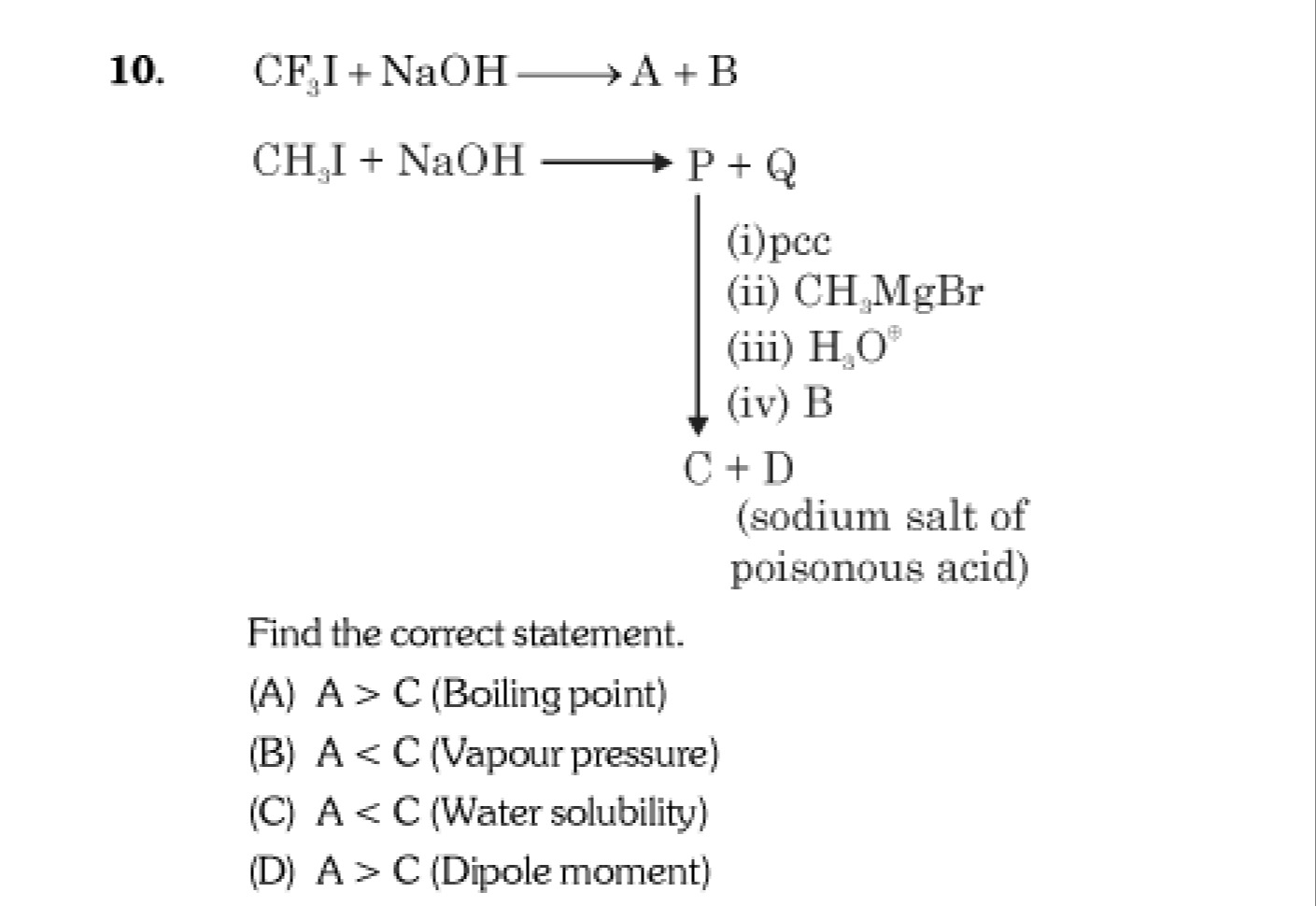
A
A>C (Boiling point)
B
A<C (Vapour pressure)
C
A<C (Water solubility)
D
A>C (Dipole moment)
Answer
A < C (Water solubility)
Explanation
Solution
Explanation of the Solution:
- The reaction of CF₃I with NaOH gives a product A that is essentially a fluorocarbon (fluoroform, HCF₃) which is very volatile and hardly soluble in water.
- In the sequence starting from CH₃I + NaOH, methanol (P) is generated and then oxidized (by PCC) to give formaldehyde. Its subsequent reaction with CH₃MgBr and work‐up, followed by the reaction with “B” (the other product from CF₃I reaction) produces an oxygenated product C (an acylated/alcohol derivative) whose structure contains polar functional groups. In addition, D is the sodium salt of trifluoroacetic acid (a very poisonous acid).
- Because product C bears polar (and hydrogen‐bonding) groups it will be more water–soluble than the non–polar compound A.
- Hence, we have A < C in water solubility.
