Question
Question: 45.4 V H₂O₂ solution (500 ml) when exposed to atmosphere looses 11.2 litre of O₂ at 1 atm, & 273 K. ...
45.4 V H₂O₂ solution (500 ml) when exposed to atmosphere looses 11.2 litre of O₂ at 1 atm, & 273 K. New molarity of H₂O₂ solution (Assume no change in volume) CT0031
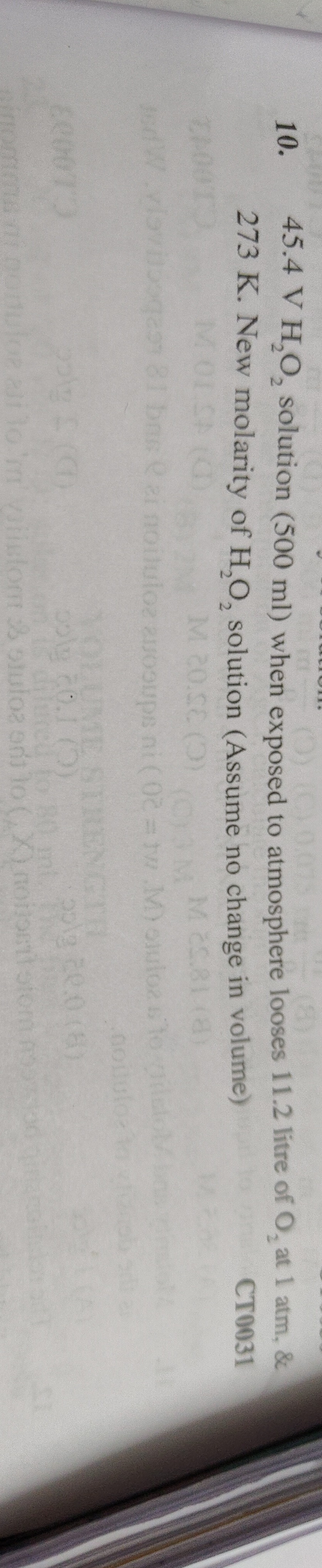
2.05 M
Solution
The problem asks for the new molarity of an H₂O₂ solution after it loses a certain volume of O₂ due to decomposition.
1. Initial Molarity of H₂O₂ Solution: The volume strength of H₂O₂ solution is given as 45.4 V. This means that 1 litre of this H₂O₂ solution will produce 45.4 litres of O₂ gas at STP (1 atm, 273 K). The decomposition of hydrogen peroxide is represented by the equation: 2H2O2(aq)→2H2O(l)+O2(g) From the stoichiometry, 2 moles of H₂O₂ produce 1 mole of O₂. At STP, 1 mole of O₂ occupies 22.4 litres. Therefore, 2 moles of H₂O₂ produce 22.4 litres of O₂ at STP. This implies that 1 mole of H₂O₂ produces 11.2 litres of O₂ at STP.
The molarity (M) of H₂O₂ solution can be calculated from its volume strength (V) using the formula: M=11.2Volume Strength Initial Molarity (Minitial) = 11.245.4=4.05357 M
2. Initial Moles of H₂O₂ in the Solution: The volume of the H₂O₂ solution is 500 ml = 0.5 L. Initial moles of H₂O₂ (ninitial) = Molarity × Volume of solution (in L) ninitial=4.05357 M×0.5 L=2.026785 moles
3. Moles of O₂ Lost: The solution loses 11.2 litres of O₂ at 1 atm and 273 K (STP conditions). Moles of O₂ lost (nO2 lost) = Molar volume at STPVolume of O₂ lost nO2 lost=22.4 L/mol11.2 L=0.5 moles
4. Moles of H₂O₂ Decomposed: From the decomposition reaction (2H2O2→2H2O+O2), 1 mole of O₂ is produced from 2 moles of H₂O₂. So, the moles of H₂O₂ decomposed (ndecomposed) = 2×nO2 lost ndecomposed=2×0.5 moles=1.0 mole
5. Remaining Moles of H₂O₂: Remaining moles of H₂O₂ (nfinal) = Initial moles - Moles decomposed nfinal=2.026785 moles−1.0 mole=1.026785 moles
6. New Molarity of H₂O₂ Solution: The problem states to assume no change in volume, so the volume of the solution remains 500 mL (0.5 L). New Molarity (Mnew) = Volume of solution (in L)Remaining moles of H₂O₂ Mnew=0.5 L1.026785 moles=2.05357 M
Rounding to three significant figures (consistent with the given 45.4 V): Mnew≈2.05 M
The final answer is 2.05 M
Explanation of the solution:
- Calculated initial molarity of H₂O₂ using the given volume strength (45.4 V) and the conversion factor (Molarity = Volume Strength / 11.2).
- Determined the initial moles of H₂O₂ in the 500 ml solution.
- Calculated the moles of O₂ lost from the solution using the given volume (11.2 L) and molar volume at STP (22.4 L/mol).
- Used the stoichiometry of H₂O₂ decomposition (2H2O2→O2) to find the moles of H₂O₂ that decomposed to produce the lost O₂.
- Subtracted the decomposed moles from the initial moles to find the remaining moles of H₂O₂.
- Calculated the new molarity by dividing the remaining moles by the original volume of the solution (assuming no volume change).
