Question
Question: Which of the following is correct order of stability of carbocation? ...
Which of the following is correct order of stability of carbocation?
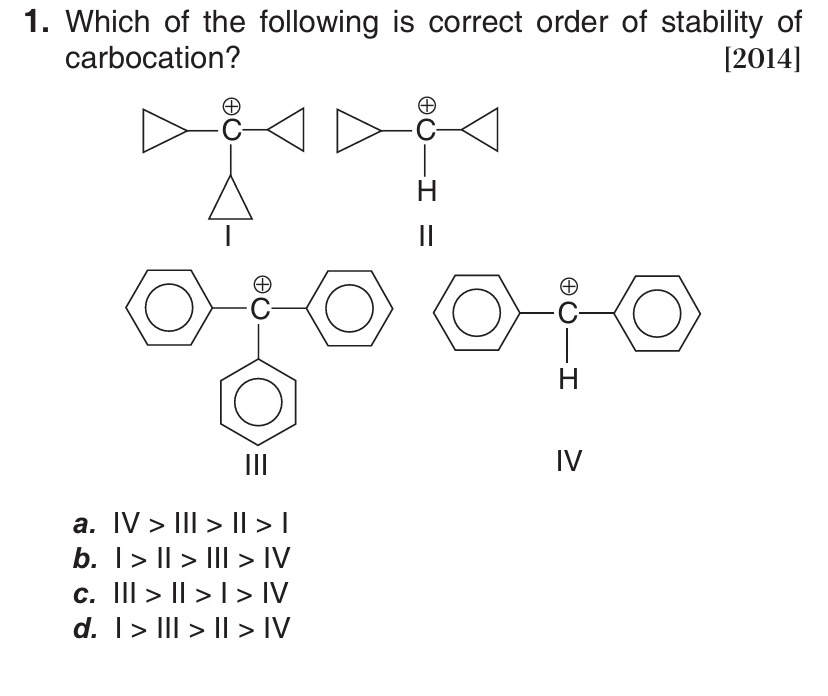
IV > III > || > |
I > II > III > IV
III > II > I > IV
I > III > II > IV
I > III > II > IV
Solution
The stability of carbocations is primarily influenced by resonance, hyperconjugation, and inductive effects. In the given structures, we have carbocations stabilized by cyclopropyl groups (σ-conjugation) and phenyl groups (resonance).
- Carbocation I is tricyclopropylmethyl carbocation. It is a tertiary carbocation stabilized by σ-conjugation from three cyclopropyl rings.
- Carbocation II is dicyclopropylmethyl carbocation. It is a secondary carbocation stabilized by σ-conjugation from two cyclopropyl rings.
- Carbocation III is triphenylmethyl carbocation. It is a tertiary carbocation stabilized by resonance from three phenyl rings.
- Carbocation IV is diphenylmethyl carbocation. It is a secondary carbocation stabilized by resonance from two phenyl rings.
Comparing tertiary and secondary carbocations with the same type of stabilizing group, tertiary is more stable than secondary. Thus, I > II and III > IV.
It is known that cyclopropylmethyl carbocation is more stable than benzyl carbocation. This suggests that σ-conjugation by a cyclopropyl group is more effective than resonance by a phenyl group.
Combining these, the correct order of stability is I > III > II > IV.
