Question
Question: 1. What is the product B of the reaction? Br $\xrightarrow[\text{Et₂O}]{\text{Mg}}$ A then, A + ...
- What is the product B of the reaction?
Br MgEt₂O A then, A +
OH
CH₃
Ο CH₂ i) Et₂Oii) H3O⁺B
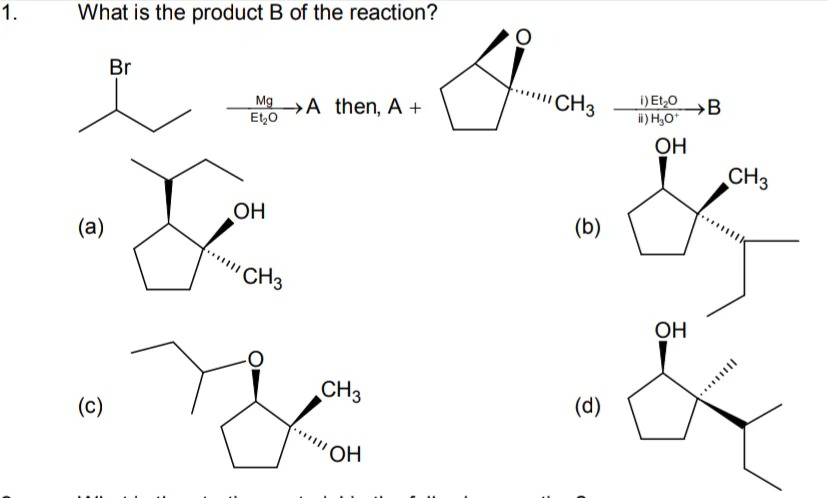
A
OH CH3
B
CH3
C
D
OH
Answer
Option (a)
Explanation
Solution
Solution Explanation
- Formation of Grignard Reagent: The bromo compound reacts with Mg in Et₂O forming the Grignard reagent (product A).
- Epoxide Opening: When A reacts with the cyclic epoxide (which has a methyl substituent on one of its two epoxide carbons), the nucleophilic isopropyl group (from A) attacks the less substituted epoxide carbon (since base‐promoted openings occur at the less hindered center). This backside attack results in inversion at that carbon.
- Work‐up: On treatment with H₃O⁺ the alkoxide is protonated to give the alcohol. Thus, the opened epoxide ends up as a cyclopentanol ring bearing three substituents:
– a hydroxyl group (–OH) at the carbon that originally bore the epoxide oxygen (now with the extra –CH₃ group),
– a methyl (–CH₃) substituent originally present on the more substituted epoxide carbon, and
– an isopropyl (–CH(CH₃)₂) group attached at the less substituted carbon (with inversion of configuration relative to the leaving group).
Comparing with the options, this matches the structure shown in option (a).
