Question
Question: The reduction potential of hydrogen electrode ($P_{H_2}$ = 1 atms; [H+] = 0.1 M) at 25°C will be -...
The reduction potential of hydrogen electrode (PH2 = 1 atms; [H+] = 0.1 M) at 25°C will be -
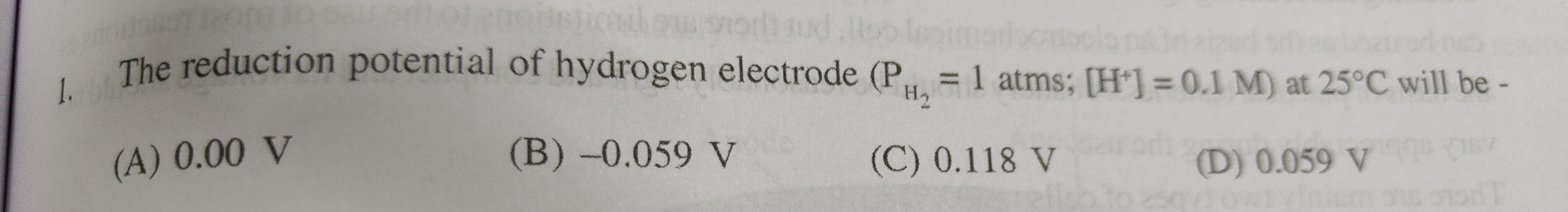
A
0.00 V
B
-0.059 V
C
0.118 V
D
0.059 V
Answer
-0.059 V
Explanation
Solution
The reduction potential is calculated using the Nernst equation: E=E0−n0.059logQ. For the hydrogen electrode, the half-reaction is H(aq)++e−→21H2(g). The standard reduction potential E0 is 0 V. The reaction quotient Q=[H+]PH21/2. Given PH2=1 atm and [H+]=0.1 M, and n=1. E=0−10.059log0.1(1)1/2=−0.059log0.11=−0.059log10=−0.059 V.
