Question
Question: The number of orbitals with n = 5, m₁ = + 2 is _____. (Round off to the Nearest Integer)....
The number of orbitals with n = 5, m₁ = + 2 is _____. (Round off to the Nearest Integer).
Answer
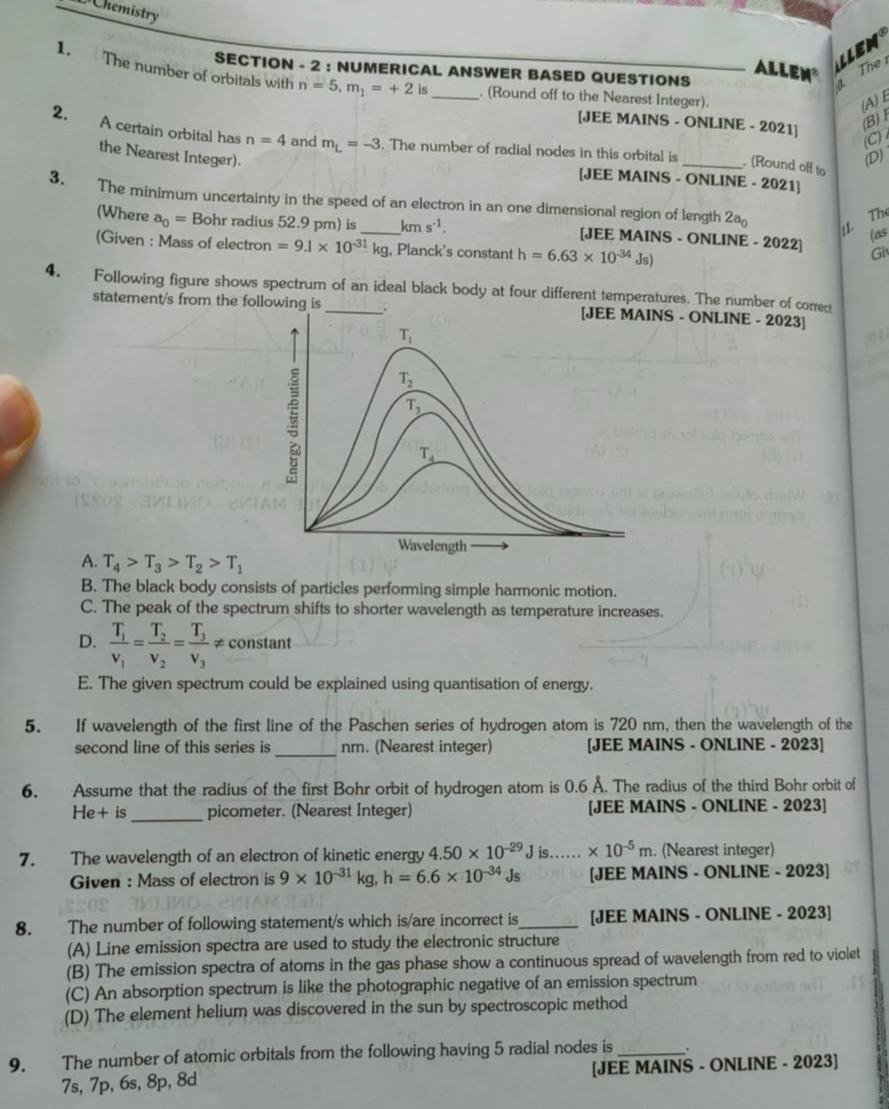
3
Explanation
Solution
For n=5, possible values of l are 0, 1, 2, 3, 4. Since m₁ = +2, l must be at least 2. Therefore, l can be 2 (5d orbital), 3 (5f orbital), or 4 (5g orbital). Each of these corresponds to one orbital with m₁=+2, giving a total of 3 orbitals.
