Question
Question: Arrange the structures according to decreasing basicity: 1 $\text{NH}_2$ 2 $\text{NH}_2$ 3 $\...
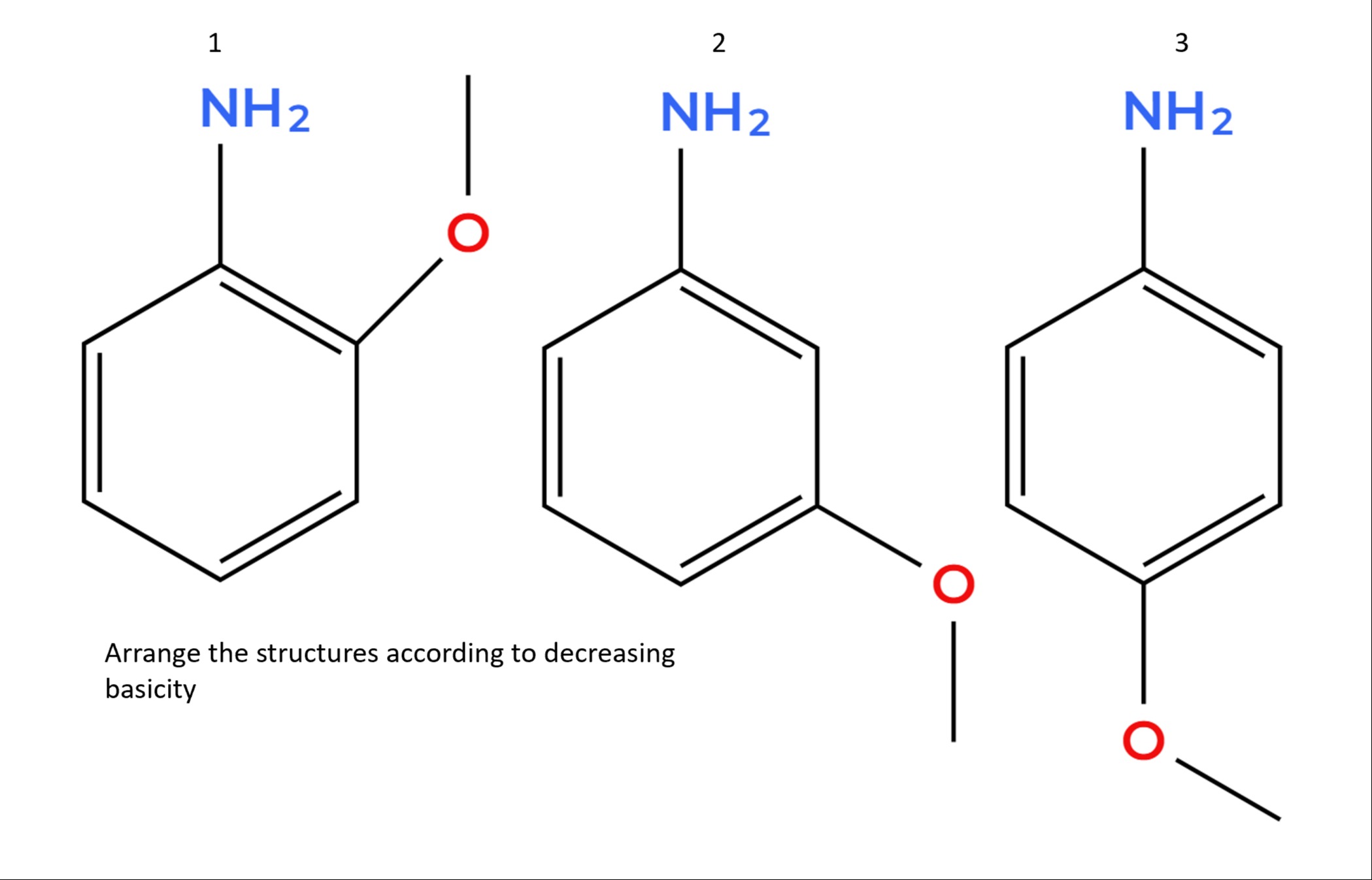
Arrange the structures according to decreasing basicity:
1 NH2
2 NH2
3 NH2
Answer
Decreasing basicity: 2 > 3 > 1
Explanation
Solution
In anilines, the lone pair on –NH₂ is delocalized into the benzene ring, reducing its basicity.
A methoxy (–OCH₃) group at the ortho and para positions can interact by resonance with the ring and further delocalize the lone pair, making it less available for protonation.
The meta position does not allow effective resonance interaction with –NH₂; only the weaker –I effect operates.
Also, the ortho substituent can form intramolecular hydrogen bonding, further lowering basicity.
Thus, the basicity order is:
Structure 2 (meta –OCH₃) > Structure 3 (para –OCH₃) > Structure 1 (ortho –OCH₃).
