Question
Question: Predict the product of the reaction. ```latex \begin{array}{ccc} & CH_3 & H\\ & | & |\\ CH_3 - C & ...
Predict the product of the reaction.
\begin{array}{ccc} & CH_3 & H\\ & | & |\\ CH_3 - C & - & C - CH_2 - CH = CH_2\\ & | & |\\ & H & Cl \end{array} \xrightarrow{alc.KOH} ?
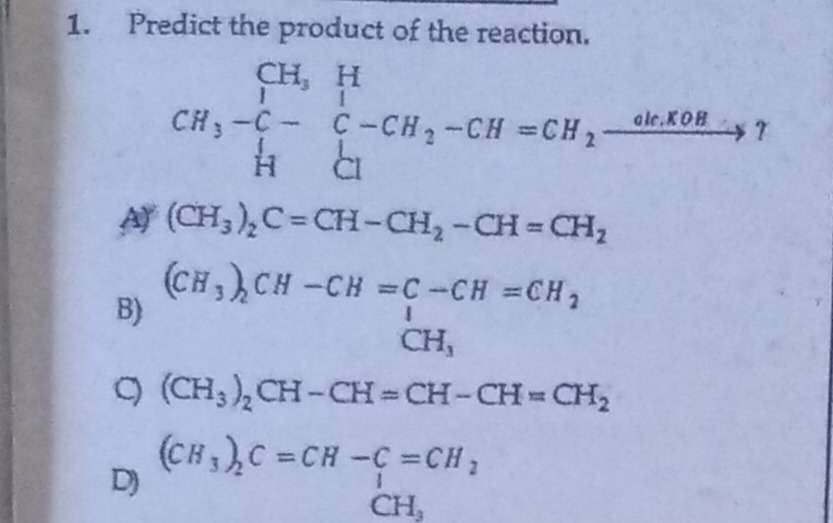
A
(CH3)2C=CH−CH2−CH=CH2
B
\begin{array}{c} (CH_3)_2CH - CH = C - CH = CH_2\\ |\\ CH_3 \end{array}
C
(CH3)2CH−CH=CH−CH=CH2
D
\begin{array}{c} (CH_3)_2C = CH - C = CH_2\\ |\\ CH_3 \end{array}
Answer
(CH3)2C=CH−CH2−CH=CH2
Explanation
Solution
We start with the compound
CH3-C(CH3)(H)-C(H)(Cl)-CH2-CH=CH2In the presence of alcoholic KOH, an E2 elimination takes place. The base abstracts a β‐hydrogen which is anti-periplanar to the chlorine. There are two possible β‑positions: one from the carbon to the left (C₂) and one from the right (C₄). Since the hydrogen on C₂ is on the more highly substituted carbon (C₂ is bonded to two methyl groups), removal of this hydrogen yields the more substituted (Zaitsev) alkene.
Thus, the elimination of H from C₂ and Cl from C₃ gives the double bond between C₂ and C₃, producing
(CH3)2C=CH−CH2−CH=CH2