Question
Question: 1-Pentyne and 2-Pentyne can be distinguished by: A.Silver mirror test B.Iodoform test C.Baeyer...
1-Pentyne and 2-Pentyne can be distinguished by:
A.Silver mirror test
B.Iodoform test
C.Baeyers test
D.Addition reaction of H2
Solution
We know that 1-pentyne and 2-pentyne are alkynes. We have to know that alkynes are unsaturated hydrocarbons which contain a minimum of one carbon-carbon triple bond. We have to know the general formula of acyclic alkynes is CnH2n−2. These compounds do not contain any other functional group other than alkynes.
Complete answer:
We are given the two alkynes namely 1-Pentyne and 2-Pentyne. We can draw the structure of 1-Pentyne as,
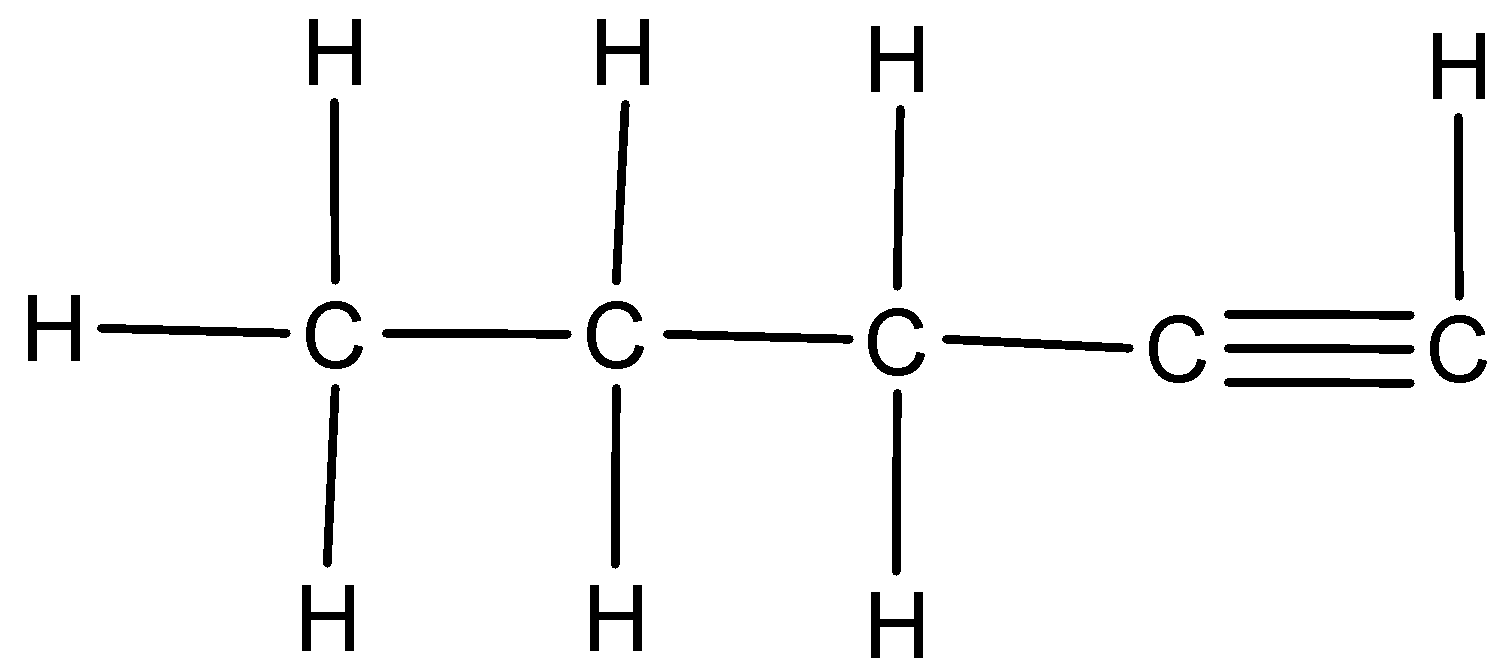 .
.
We can draw the structure of 2-Pentyne as,
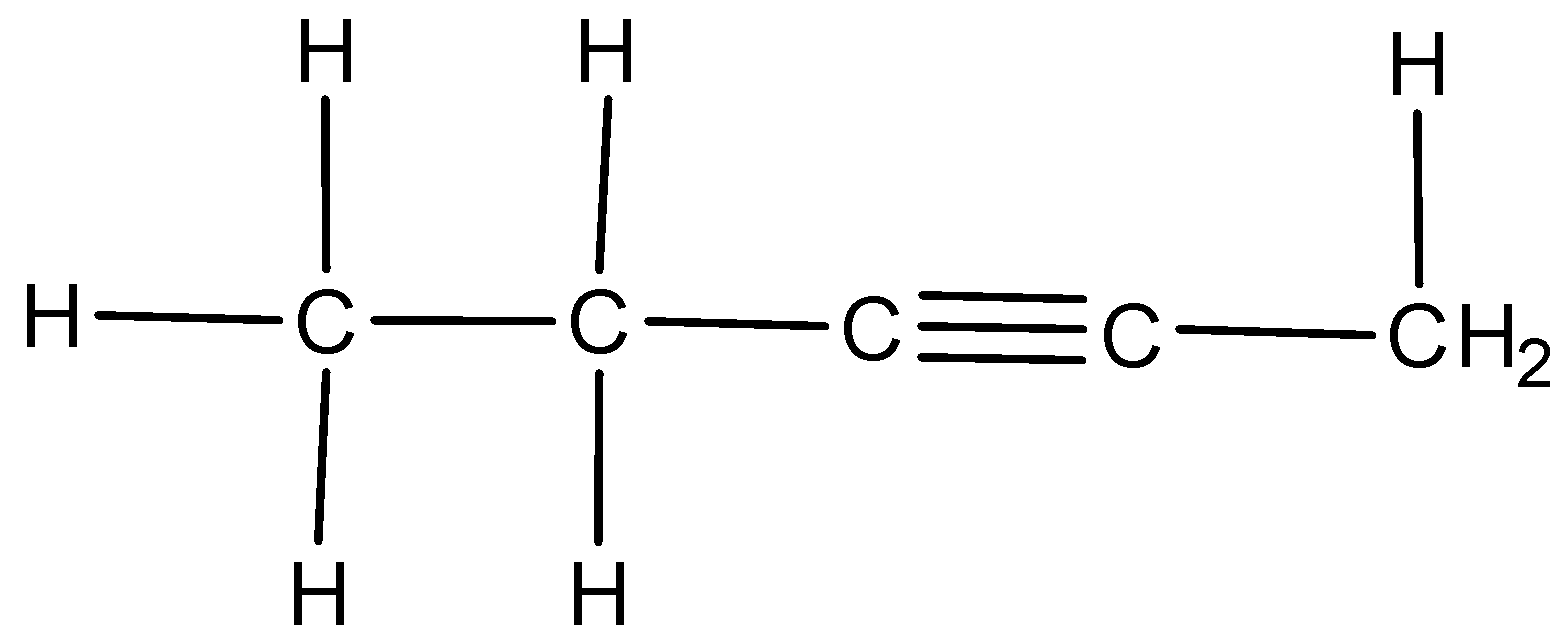
Because of the presence of two pi-bonds, a number of additional reactions are undergone by alkynes. In these reactions, two molecules of reagent could be added to alkynes whereas in alkenes only one molecule of reagent could be added to alkene because of the presence of one pi-bond.
We can distinguish 1-Pentyne and 2-Pentyne through the silver mirror test. We can perform a silver mirror test through Tollen’s reagent. We have to know that Tollen’s reagent is a chemical reagent that contains silver nitrate solution, ammonia solution and a certain amount of sodium hydroxide. Because of the acidic nature of atoms of hydrogen bonded to carbon that is triple bonded. We have to know that terminal alkynes react with metallic salt that is heavy such as silver nitrate.
We have to know that 1-pentyne is a terminal alkyne; it would react with Tollen’s reagent whereas 2-pentyne would not react with Tollen’s reagent because 2-pentyne is an internal alkyne.
So, we can distinguish 1-pentyne and 2-pentyne through the Silver mirror test.
Option (A) is correct.
Note:
We have to know that alkynes containing terminal carbon-carbon triple bond would be treated with an ammoniacal solution of silver nitrate to form white precipitate of silver acetylide. We have to know that Tollen’s reagent would react with terminal alkyne because they contain acidic hydrogen, so they are known as the silver mirror test.
