Question
Question: Observe the following coloumn and answer the following questions. Classify the following groups as +...
Observe the following coloumn and answer the following questions. Classify the following groups as +m, -m, +I, -I, +HC, -HC.
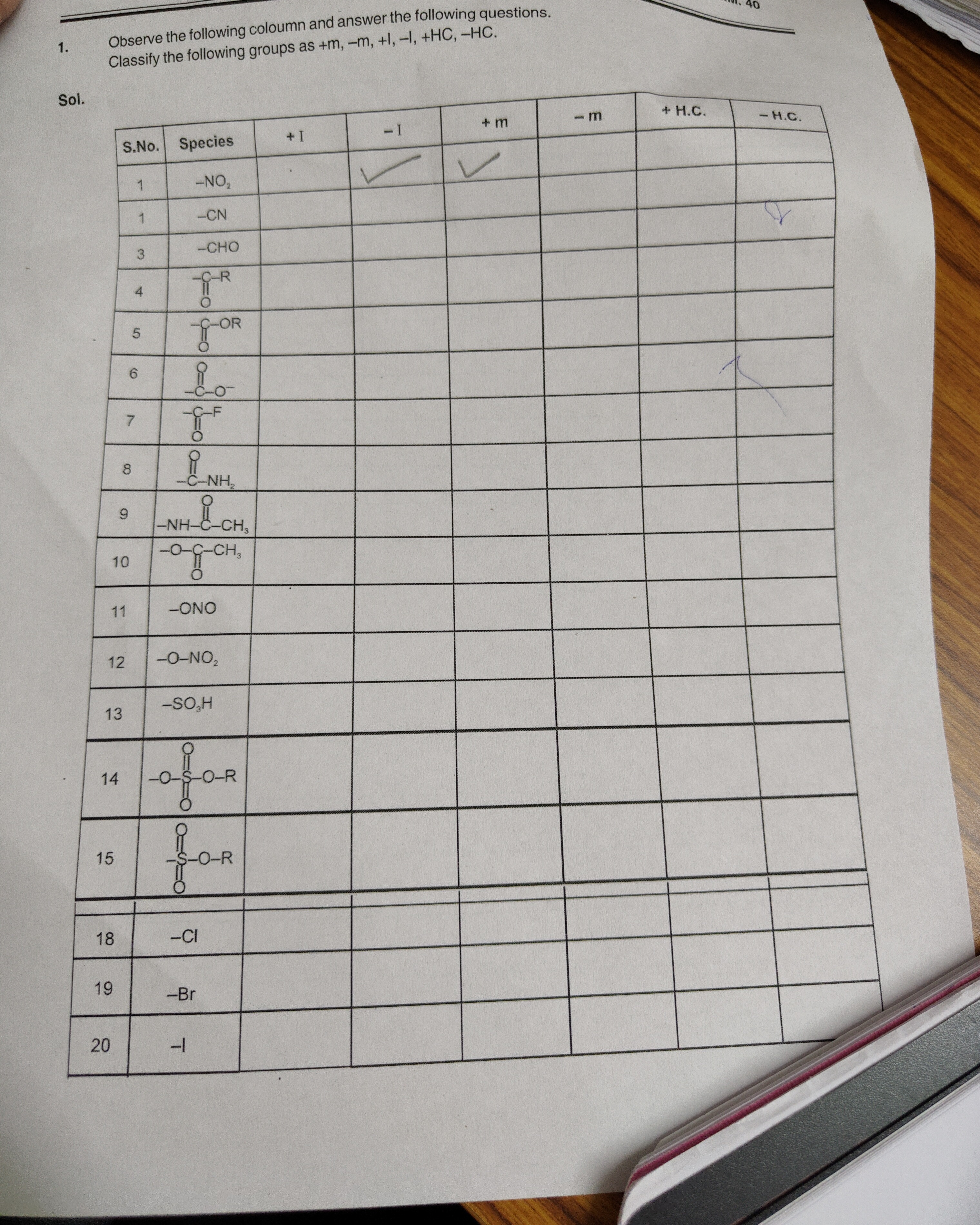
-NO₂
-CN
-CHO
-COR
-CO-
-CF
-CNH₂
-NHCOCH₃
-OCOCH₃
-ONO
-ONO₂
-SO₃H
-OSOR
-SOR
-Cl
-Br
-I
The question asks to classify functional groups based on their electronic effects. The classification for each group is as follows:
- -NO₂: -I, -m
- -CN: -I, -m
- -CHO: -I, -m
- -COR: -I, -m
- -CO-: -I, -m
- -CF: -I (primarily)
- -CNH₂ (assuming -CONH₂): -I, -m
- -NHCOCH₃: +m, -I
- -OCOCH₃: +m, -I
- -ONO: -I, -m
- -ONO₂: -I, -m
- -SO₃H: -I, -m
- -OSOR: -I, -m
- -SOR: -I, -m
- -Cl: -I, +m
- -Br: -I, +m
- -I: -I, +m
Solution
The classification of each group is determined by its electronic structure and the nature of the atom directly attached to the pi system. Inductive effects (-I/+I) are based on electronegativity and hybridization, while mesomeric effects (-m/+m) are based on resonance delocalization of electrons. Hyperconjugation (+HC) involves delocalization of sigma electrons. Standard definitions are applied, noting that -HC is not a standard classification. Groups like -NO₂, -CN, -CHO, -COR, -CO-, -SO₃H, -SOR, -ONO, -ONO₂, -OSOR are electron-withdrawing via -I and/or -m effects. Groups like -NHCOCH₃, -OCOCH₃ exhibit +m effects due to lone pairs on N/O, alongside -I effects. Halogens (-Cl, -Br, -I) show both -I and weak +m effects, with -I dominating.
