Question
Question: 1 mol of ideal gas is expanded from (10 atm, 10 L) to (2 atm, 50 L) isothermally. Calculate: q, $\De...
1 mol of ideal gas is expanded from (10 atm, 10 L) to (2 atm, 50 L) isothermally. Calculate: q, ΔU, ΔH, W.
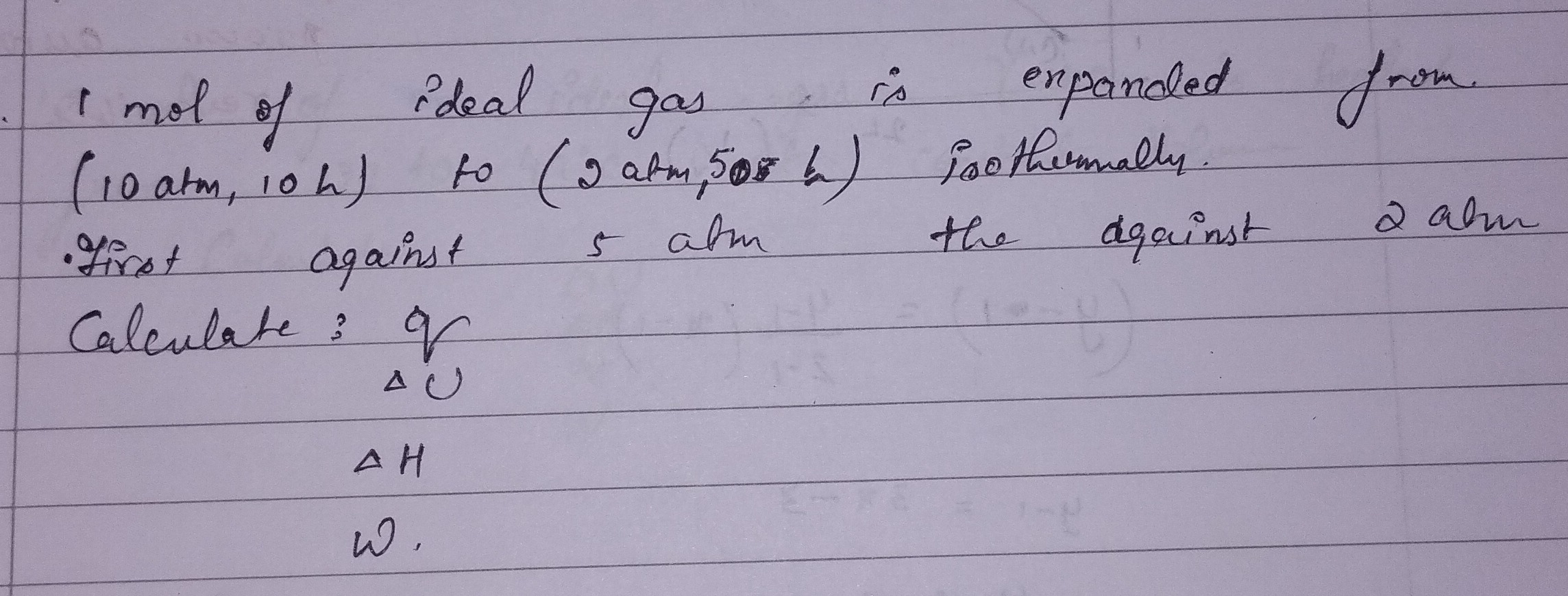
q = 11.15 kJ, ΔU = 0 kJ, ΔH = 0 kJ, W = -11.15 kJ
q = 0 kJ, ΔU = 11.15 kJ, ΔH = 0 kJ, W = -11.15 kJ
q = 11.15 kJ, ΔU = 0 kJ, ΔH = 11.15 kJ, W = 0 kJ
q = 0 kJ, ΔU = 0 kJ, ΔH = 0 kJ, W = -11.15 kJ
q = 11.15 kJ, ΔU = 0 kJ, ΔH = 0 kJ, W = -11.15 kJ
Solution
For an isothermal process of an ideal gas, ΔU=0 and ΔH=0. The work done in an isothermal expansion is given by W=−nRTln(V1V2). Given: n=1 mol, T is constant, P1=10 atm, V1=10 L, P2=2 atm. Using Boyle's Law for an isothermal process: P1V1=P2V2. 10 atm×10 L=2 atm×V2 V2=2100=50 L.
The work done is W=−PextΔV. The problem states expansion against 5 atm then against 2 atm, indicating an irreversible, multi-stage expansion.
Stage 1: Expansion from V1=10 L to an intermediate volume Vint against Pext,1=5 atm. The gas pressure drops until it equals the external pressure. Using Boyle's Law for the gas itself: P1V1=Pgas,intVint 10 atm×10 L=5 atm×Vint Vint=5100=20 L. Work done in Stage 1: W1=−Pext,1(Vint−V1)=−5 atm×(20 L−10 L)=−5 atm×10 L=−50 atm L.
Stage 2: Expansion from Vint=20 L to V2=50 L against Pext,2=2 atm. Work done in Stage 2: W2=−Pext,2(V2−Vint)=−2 atm×(50 L−20 L)=−2 atm×30 L=−60 atm L.
Total Work: W=W1+W2=−50 atm L+(−60 atm L)=−110 atm L.
Convert work to Joules: 1 atm L=101.325 J. W=−110×101.325 J=−11145.75 J≈−11.15 kJ.
From the First Law of Thermodynamics, ΔU=q+W. Since ΔU=0 for an isothermal process of an ideal gas, 0=q+W, so q=−W. q=−(−110 atm L)=110 atm L. q=110×101.325 J=11145.75 J≈11.15 kJ.
Therefore: q≈11.15 kJ ΔU=0 kJ ΔH=0 kJ W≈−11.15 kJ
