Question
Question: Identify reactions correctly matched with their major product?...
Identify reactions correctly matched with their major product?
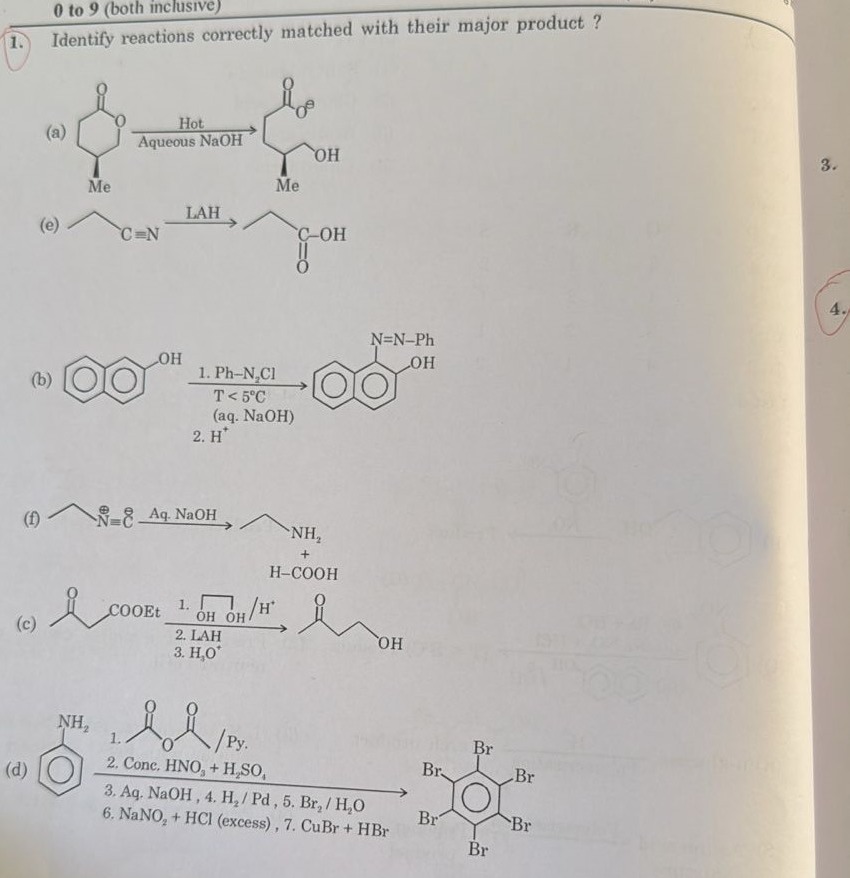
(a)
(e)
(b)
(f)
(c)
(d)
a, b, c, f
Solution
The question asks to identify the reactions correctly matched with their major product. We need to analyze each reaction.
(a) The reactant is 4-methyltetrahydro-2H-pyran-2-one, which is a cyclic ester (lactone). Reaction with hot aqueous NaOH is basic hydrolysis of the ester. This opens the ring, forming a carboxylate salt and an alcohol. The product shown is 6-hydroxy-4-methylhexanoate, which is the correct product of hydrolysis of 4-methyltetrahydro-2H-pyran-2-one in basic conditions. Thus, reaction (a) is correctly matched.
(b) The reactant is 2-naphthol (β-naphthol). Reaction with benzenediazonium chloride in aqueous NaOH at low temperature is an azo coupling reaction. Electrophilic aromatic substitution occurs at the position ortho or para to the activating group (OH). For 2-naphthol, positions 1 and 3 are ortho/para to the OH group. Position 1 is generally more reactive than position 3. The product shown is 1-phenylazo-2-naphthol, where the azo group is attached at position 1 of 2-naphthol. This is the correct major product of this azo coupling reaction. Thus, reaction (b) is correctly matched.
(c) The reactant is ethyl 3-oxobutanoate (ethyl acetoacetate). The reaction sequence involves: 1. Ethylene glycol/H+, 2. LAH, 3. H3O+.
- Step 1: Reaction with ethylene glycol in the presence of acid forms a cyclic ketal from the ketone group. The ester group remains unchanged.
- Step 2: Reduction with LAH (Lithium Aluminum Hydride). LAH reduces esters to primary alcohols. The ketal is resistant to LAH reduction. So, the ester group is reduced to a primary alcohol.
- Step 3: Hydrolysis with H3O+ cleaves the ketal, regenerating the ketone group.
The product is 4-hydroxybutan-2-one. The product shown in the question is 4-hydroxybutan-2-one. Thus, reaction (c) is correctly matched.
(d) The reactant is aniline. The reaction sequence is: 1. Acetic anhydride/Pyridine, 2. Conc. HNO3/H2SO4, 3. Aq. NaOH, 4. H2/Pd, 5. Br2/H2O, 6. NaNO2 + HCl (excess), 7. CuBr + HBr.
- Step 1: Acetylation of aniline to form acetanilide.
- Step 2: Nitration of acetanilide. The acetamido group is an ortho, para director. The major product is p-nitroacetanilide.
- Step 3: Hydrolysis of acetanilide to form p-nitroaniline.
- Step 4: Reduction of the nitro group to an amino group using H2/Pd. This gives p-phenylenediamine (benzene-1,4-diamine).
- Step 5: Bromination with bromine water. Amino groups are strongly activating and direct bromination to ortho and para positions. In p-phenylenediamine, both amino groups are activating. The positions ortho to one amino group are also ortho to the other, and similarly for para. The positions ortho to both amino groups are positions 2, 3, 5, and 6. All these positions are activated for electrophilic substitution. Bromination with bromine water typically leads to polysubstitution on strongly activated rings. Therefore, all available ortho and para positions to the amino groups will be substituted. In p-phenylenediamine, positions 2, 3, 5, and 6 are all ortho or para to the amino groups. Therefore, tetrabromination is expected, leading to 2,3,5,6-tetrabromo-1,4-diaminobenzene. The product shown is hexabromobenzene. This step does not produce hexabromobenzene.
- Step 6 & 7: Diazotization of the amino groups and Sandmeyer reaction. Diazotization of the amino groups in 2,3,5,6-tetrabromo-1,4-diaminobenzene followed by reaction with CuBr/HBr would replace the diazonium groups with bromine atoms. This would lead to hexabromobenzene. However, the product of step 5 is 2,3,5,6-tetrabromo-1,4-diaminobenzene, not aniline. The reaction sequence from aniline to hexabromobenzene would involve bromination first to get aniline with some bromine substituents, followed by diazotization and other steps. The given sequence leads to p-phenylenediamine before bromination. The product shown, hexabromobenzene, is not the major product of this entire reaction sequence starting from aniline. Thus, reaction (d) is incorrectly matched.
(e) The reactant is butanenitrile. Reaction with LAH is reduction of the nitrile group to a primary amine. Nitriles are reduced to primary amines by LAH.
CH3CH2CH2C≡N+LAH→CH3CH2CH2CH2NH2.
The product shown is butanoic acid (CH3CH2CH2COOH). This is incorrect. Reduction of butanenitrile with LAH yields butan-1-amine. Thus, reaction (e) is incorrectly matched.
(f) The reactant is ethyl isocyanide (ethyl isonitrile), CH3CH2N≡C. Reaction with aqueous NaOH is alkaline hydrolysis of isocyanides. Hydrolysis of isocyanides yields a primary amine and formic acid.
CH3CH2N≡C+2H2ONaOHCH3CH2NH2+HCOOH.
The products shown are ethylamine (CH3CH2NH2) and formic acid (H-COOH). Thus, reaction (f) is correctly matched.
Based on the analysis, reactions (a), (b), (c), and (f) are correctly matched with their major products. The question asks to identify reactions correctly matched with their major product. Since multiple options can be correct, we list all the correct ones.
