Question
Question: For the reaction: xA $\rightarrow$ yB, $\log_{10}(-\frac{d[A]}{dt}) = \log_{10}(+\frac{d[B]}{dt})+0....
For the reaction: xA → yB, log10(−dtd[A])=log10(+dtd[B])+0.3
If the value of log105=0.7, the value of x : y is :
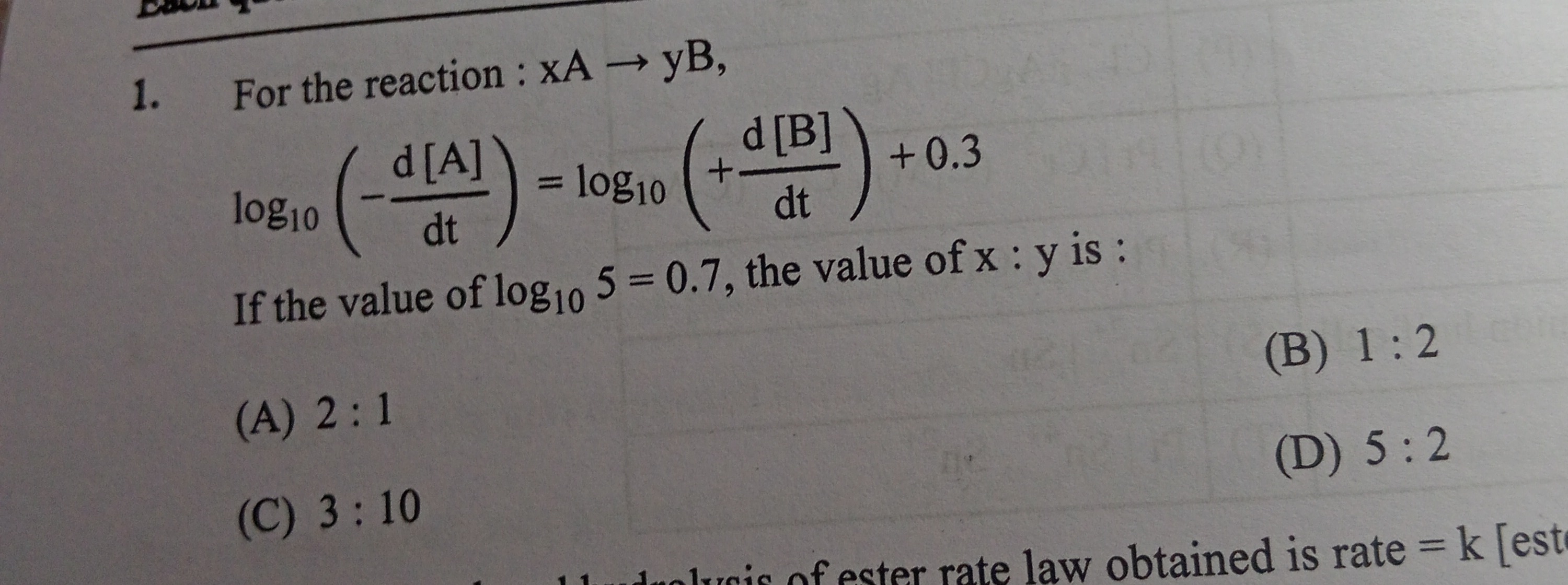
2 : 1
1 : 2
3 : 10
5 : 2
2 : 1
Solution
The rate of the reaction xA → yB can be expressed in terms of the rate of disappearance of A and the rate of appearance of B using the stoichiometric coefficients:
Rate =−x1dtd[A]=+y1dtd[B]
From this relationship, we can write:
−dtd[A]=yx(+dtd[B])
The problem gives a logarithmic relationship between the rates:
log10(−dtd[A])=log10(+dtd[B])+0.3
Substitute the expression for −dtd[A] from the stoichiometric relationship into the given equation:
log10(yx(+dtd[B]))=log10(+dtd[B])+0.3
Using the logarithm property log(AB)=logA+logB:
log10(yx)+log10(+dtd[B])=log10(+dtd[B])+0.3
Subtract log10(+dtd[B]) from both sides of the equation:
log10(yx)=0.3
We are given that log105=0.7. We know that log1010=1. Using the logarithm property log(A/B)=logA−logB:
log102=log10(510)=log1010−log105=1−0.7=0.3
So, the constant 0.3 is equal to log102. Substitute this back into the equation for yx:
log10(yx)=log102
Since the logarithms are equal and the base is the same, the arguments must be equal:
yx=2
This means the ratio x : y is 2 : 1.
