Question
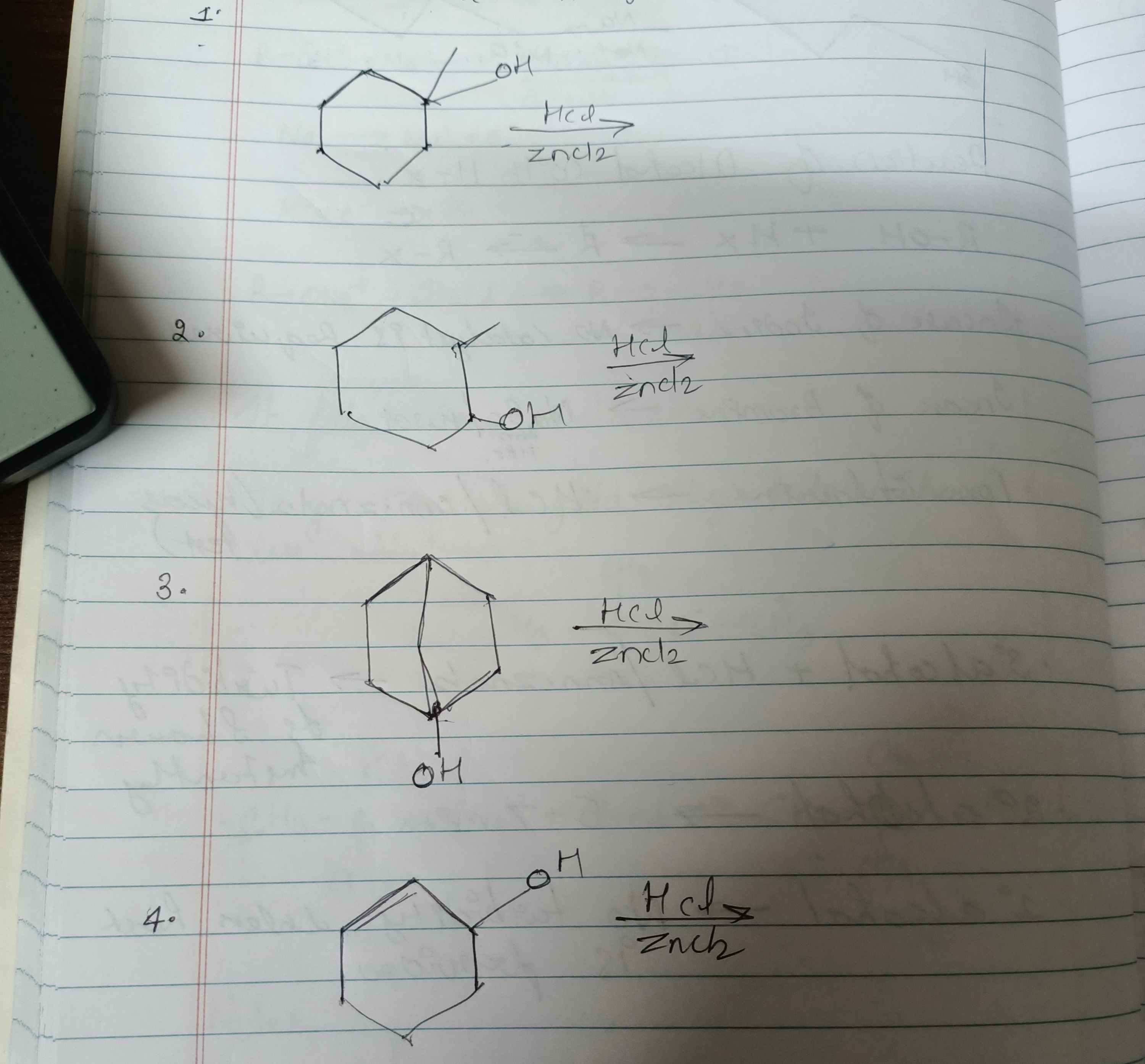
Question: Which of the following alcohols will react rapidly with Lucas reagent (HCl/ZnCl$_2$)? 1. 1-methylc...
Which of the following alcohols will react rapidly with Lucas reagent (HCl/ZnCl2)?
- 1-methylcyclohexan-1-ol
- Cyclohexanol
- Bicyclic Tertiary Alcohol
- Cyclohex-2-en-1-ol
A
1, 3, and 4
B
1 and 3 only
C
2 and 4 only
D
1, 2, 3, and 4
Answer
1, 3, and 4
Explanation
Solution
The reagent HCl/ZnCl2 is known as Lucas reagent, which is used to differentiate between primary, secondary, and tertiary alcohols based on their reaction rates.
- Tertiary alcohols react rapidly with Lucas reagent to form alkyl chlorides, causing immediate turbidity.
- Secondary alcohols react slower, typically forming turbidity within 5-20 minutes, sometimes requiring gentle heating.
- Primary alcohols react very slowly or not at all.
- Allylic and benzylic alcohols react rapidly because the carbocation intermediates formed are resonance-stabilized, similar to tertiary alcohols.
Analyzing each structure:
- 1-methylcyclohexan-1-ol: This is a tertiary alcohol. It reacts rapidly with Lucas reagent.
- Cyclohexanol: This is a secondary alcohol. It reacts slowly with Lucas reagent.
- Bicyclic Tertiary Alcohol: The carbon atom bearing the hydroxyl group is bonded to three other carbon atoms, classifying it as a tertiary alcohol. It reacts rapidly with Lucas reagent.
- Cyclohex-2-en-1-ol: This is a secondary alcohol that is also allylic to the double bond. The formation of a resonance-stabilized allylic carbocation leads to a rapid reaction with Lucas reagent, comparable to tertiary alcohols.
Therefore, alcohols 1, 3, and 4 react rapidly with HCl/ZnCl2.
