Question
Question: 1) Explain the limitations of Octet rule. 2) How many electrons will be around \[I\](iodine) in th...
- Explain the limitations of Octet rule.
- How many electrons will be around I(iodine) in the compound IF7.
Solution
Iodine heptafluoride (IF7 )is an interhalogen compound, a compound formed by one halogen reacting with another halogen. The halogen-halogen bond in interhalogen compounds is weaker than that of bonds in di-halogen molecules. By constructing the Lewis structure, we find the number of electrons will be around I(iodine) in the compound IF7.
Complete answer:
1)
Limitations of Octet rule: 1. Hydrogen with one electron attains stability by sharing, gaining or losing one valence electron. It does not need to complete octet to attain stability. Also, He has only 2 electrons and is stable. 2. Incomplete octet: In certain molecules such as BeH2, BeCl2, BH3, BF3, the central atom has less than 8 electrons in its valence shell, yet the molecule is stable. 3. Expanded octet: In certain molecules such as PF5,SF6, IF7, H2SO4, the central atom has more than 8 valence electrons, yet the molecule is stable.
Explanation:
One limitation of the octet rule is that it cannot be applied to the nonmetals after silicon in the periodic table.
These elements can “expand their octet” and have more than eight valence electrons around the central atom.
Examples are PF5,SF6, and H2SO4.
The P atom in PF5 has 10 electrons in its valence shell:
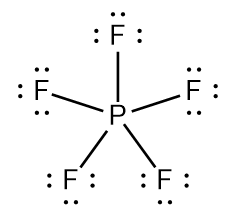
The S atom in SF6 has 12 electrons in its valence shell:
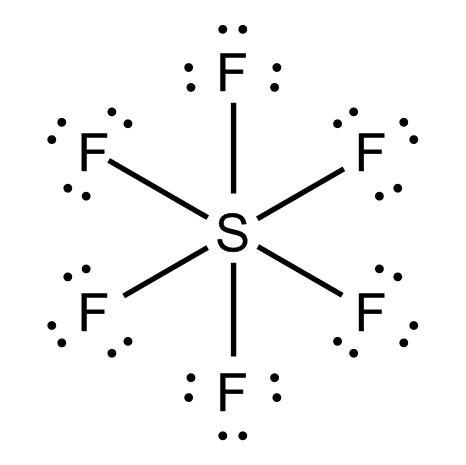
And so does the S atom in H2SO4.
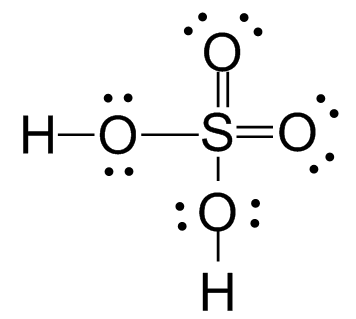
Molecules with an odd number of electrons such as NO and NO2 cannot satisfy the octet rule. One atom must have an odd number of electrons. The N atom in NO has only 7 electrons in its valence shell. In NO2, one of the O atoms has only 7 electrons in its valence shell.
In some molecules the central atom cannot possibly have eight valence electrons. For example, BeCl2 and BCl3 do not obey the octet rule. Be has only 4 electrons in its valence shell and B has only 6 electrons in its valence shell.
The Lewis structure of IF7:
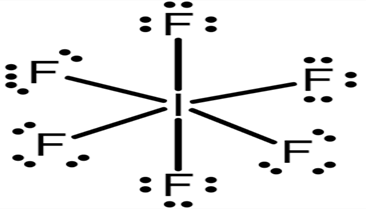
The I(iodine) atom in the compound IF7 is surrounded by 12 electrons (six pairs of electrons) yet it is stable.
Note:
Note that in octet rule, atoms can combine either by transferring or by sharing their valence electrons to attain an octet of electrons in their valence shell. IF7 is a colourless gas. It is prepared by reaction iodine pentafluoride with fluorine gas. Halogens are extremely reactive in nature as they require only electrons in their valence shell to complete their octet.
