Question
Question: 1\. Define standard enthalpy of combustion. 2\. calculate the enthalpy change for the reaction: ...
1. Define standard enthalpy of combustion.
2. calculate the enthalpy change for the reaction:
N2(g)+3H2(g)→2NH3(g)
The bond enthalpies are:
Bond 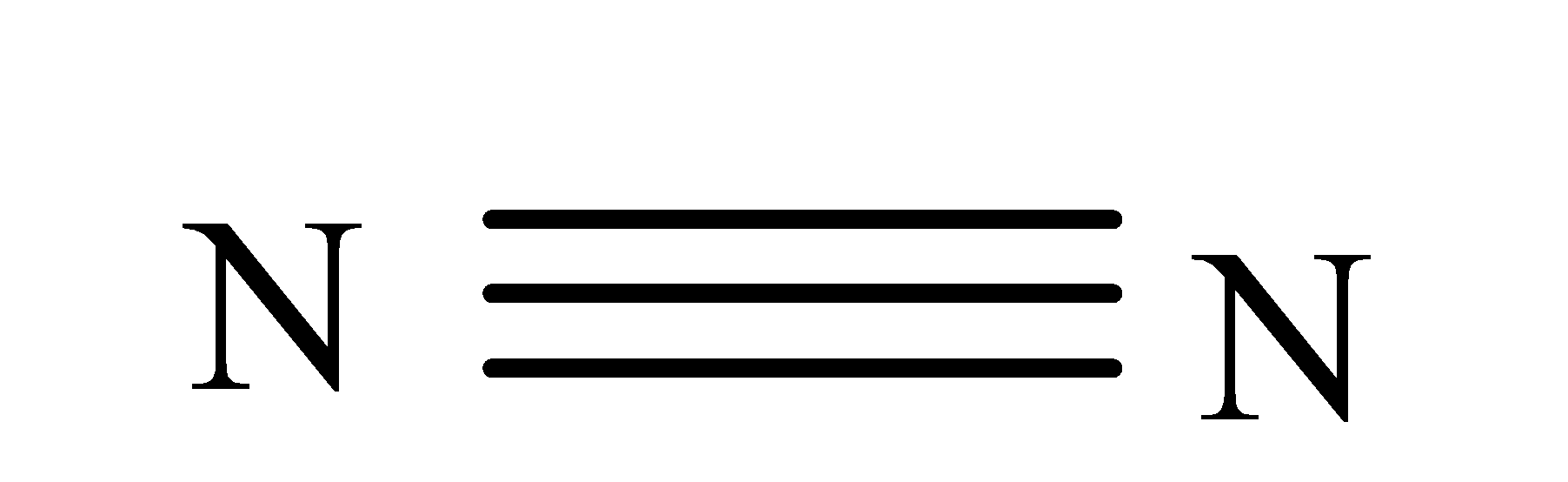 H-H N-H
H-H N-H
ΔH∘kJmole−1 946 435 389
Solution
By combustion we mean any substance which burns in the presence of excess of air and by standard state we mean the standard conditions of temperature and pressure i.e. 1 atm pressure and 273K temperature. And by the enthalpy change we means the amount of heat released or absorbed when pressure is constant and can be calculated by using the formula as; Enthalpy change ΔH∘=∑ΔH( reactants)-∑ΔH( products). Now answer the statement.
Complete answer:
1. By the term standard enthalpy of combustion we means the change produced in the enthalpy when one mole of the substance or any compound is burnt in the presence of excess of oxygen and all the reactants and products are present in their standard states i.e. in their gaseous states and under standard conditions of temperature and pressure i.e. 1 atm pressure and 273 K temperature. Example:
C3H8(g)+5O2(g)→3CO2(g0+4H2O(g)
Now coming to the second part.
2.First of all. Let’s discuss what enthalpy changes . By the enthalpy change we means the amount of heat taken or given out in any reaction which occurs at the constant pressure and it denoted by the symbol asΔH∘and its symbols are kJmole−1 .
The bond enthalpies of the reaction are as;
N2(g)+3H2(g)→2NH3(g)
Bond enthalpy of ammonia ΔH∘kJmole−1 = 389kJmole−1
Bond enthalpy of Nitrogen ΔH∘kJmole−1 = 946kJmole−1
Bond enthalpy of hydrogen ΔH∘kJmole−1 = 435kJmole−1
So, now the enthalpy change for the reaction can be calculated as;
Enthalpy change ΔH∘=∑ΔH( reactants)-∑ΔH( products) =∑ΔHN2+∑3ΔHH2−∑6ΔHNH3 = 946+3×435-6×389 = 946+ 1305-2334 =- 83 kJ mole−1
Therefore, the enthalpy changes ΔH∘ for the reaction is -83kJmole−1
Note:
To calculate the enthalpy change always takes the units in their standard states and under standard conditions of temperature and pressure and if the substances are present in the solid state, then their enthalpy is always taken as zero.
