Question
Question: Consider the following heat engine involving one mole of ideal monatomic gas. The gas begins at temp...
Consider the following heat engine involving one mole of ideal monatomic gas. The gas begins at temperature T0, pressure P0, and volume V0, and undergoes four reversible steps.
- The gas is expanded at constant pressure until its
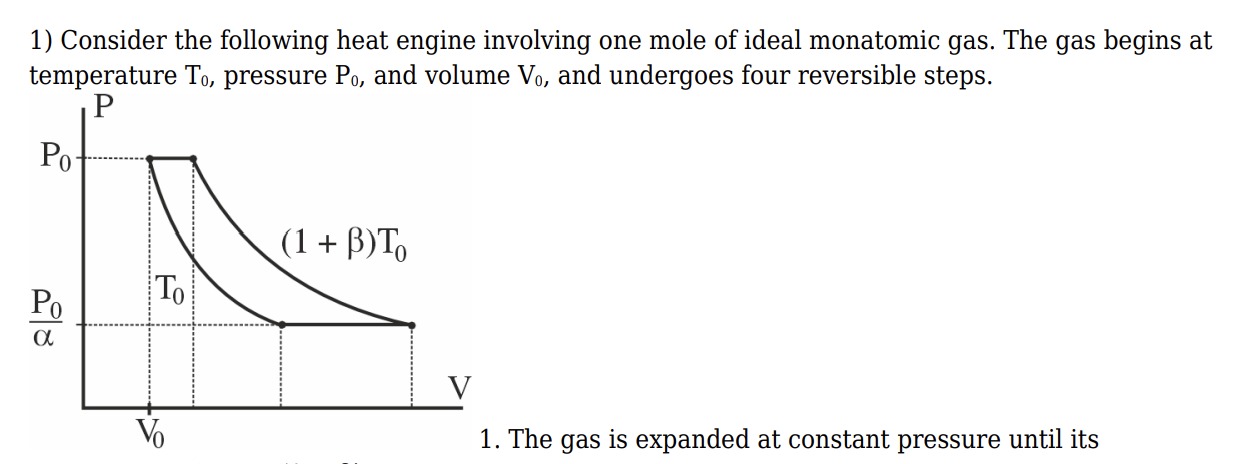
temperature is (1+\beta)T_0
Solution
The provided question is incomplete. It asks to complete the sentence describing the first step of the heat engine cycle. Based on the diagram and the description of the initial state and the nature of the first step, the first step is a constant pressure expansion from state 1 to state 2.
State 1: (P0,V0,T0).
The first step is at constant pressure P0. From the diagram, the process goes from temperature T0 to (1+β)T0.
Using the ideal gas law PV=nRT, with n=1, we have P0V0=RT0.
Let state 2 be (P2,V2,T2). For the first step, P2=P0 and T2=(1+β)T0.
From the ideal gas law at state 2, P2V2=RT2, so P0V2=R(1+β)T0.
Dividing by P0V0=RT0, we get V0V2=T0(1+β)T0=1+β.
So, V2=(1+β)V0.
The first step is a constant pressure expansion from (P0,V0,T0) to (P0,(1+β)V0,(1+β)T0).
The sentence "1. The gas is expanded at constant pressure until its" can be completed by stating either the final volume or the final temperature.
Possible completions are:
-
volume is (1+β)V0.
-
temperature is (1+β)T0.
Since the options are not given, we cannot select the correct option. However, if the question asks for the description of the first step, either of the above completions would be appropriate. Assuming a multiple choice question, one of these would likely be an option.
