Question
Question: Calculate the potential of Iron electrode in which the concentration of Fe2+ ion is 0.01 M. (2024) ...
Calculate the potential of Iron electrode in which the concentration of Fe2+ ion is 0.01 M. (2024)
(E°Fe2+/Fe = 0.45 V at 298 K)
[Given: log 10 = 1]
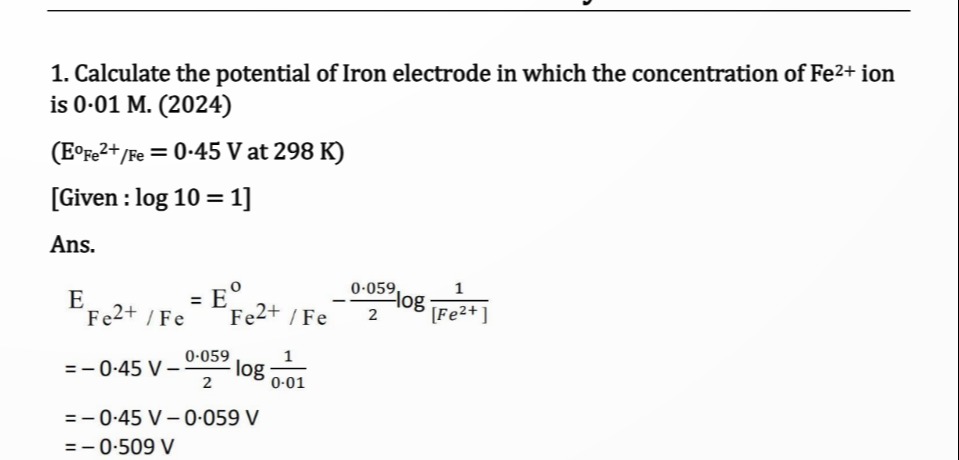
EFe2+ / Fe = -0.509 V
Solution
The problem requires calculating the electrode potential of an Iron electrode under non-standard conditions using the Nernst equation.
1. Identify the half-reaction and number of electrons (n): The reduction half-reaction for the iron electrode is: Fe2+(aq)+2e−→Fe(s) From this, the number of electrons involved, n=2.
2. Identify the given values:
- Concentration of Fe²⁺, [Fe2+]=0.01 M
- Standard electrode potential, EFe2+/Fe∘=0.45 V (as stated in the question). However, the provided solution uses −0.45 V. Standard reduction potential for Fe2+/Fe is typically negative (around −0.44 V to −0.45 V). Given that the provided solution proceeds with −0.45 V and arrives at a chemically plausible result, we will assume EFe2+/Fe∘=−0.45 V for consistency with the provided solution's calculation.
- Temperature, T=298 K (implied by the use of 0.059 in the Nernst equation).
- Given: log10=1.
3. Apply the Nernst equation: The Nernst equation for a reduction half-reaction Mn+(aq)+ne−→M(s) at 298 K is: EMn+/M=EMn+/M∘−n0.059log[Mn+]1
Substitute the values for the Iron electrode: EFe2+/Fe=EFe2+/Fe∘−20.059log[Fe2+]1 EFe2+/Fe=−0.45 V−20.059log0.011
4. Calculate the logarithmic term: log0.011=log10011=log100 Since 100=102, we have: log100=log(102)=2log10 Given log10=1, log100=2×1=2
5. Complete the calculation: Substitute the value of the logarithmic term back into the Nernst equation: EFe2+/Fe=−0.45 V−20.059×2 EFe2+/Fe=−0.45 V−0.059 V EFe2+/Fe=−0.509 V
The calculated potential of the Iron electrode is −0.509 V.
Explanation of the solution:
The electrode potential for the Iron electrode is calculated using the Nernst equation.
- Reaction: Fe2+(aq)+2e−→Fe(s). Here, n=2.
- Nernst Equation: E=E∘−n0.059log[Fe2+]1 at 298 K.
- Substitute values: E∘=−0.45 V (assuming this value, consistent with the provided solution's calculation), n=2, [Fe2+]=0.01 M. E=−0.45−20.059log0.011
- Calculate log term: log0.011=log100=2.
- Final Calculation: E=−0.45−20.059×2=−0.45−0.059=−0.509 V.
Answer:
The potential of the Iron electrode is -0.509 V.
