Question
Question: An ideal gas expands isothermally along ab and does 600 J of work. During the process :- ...
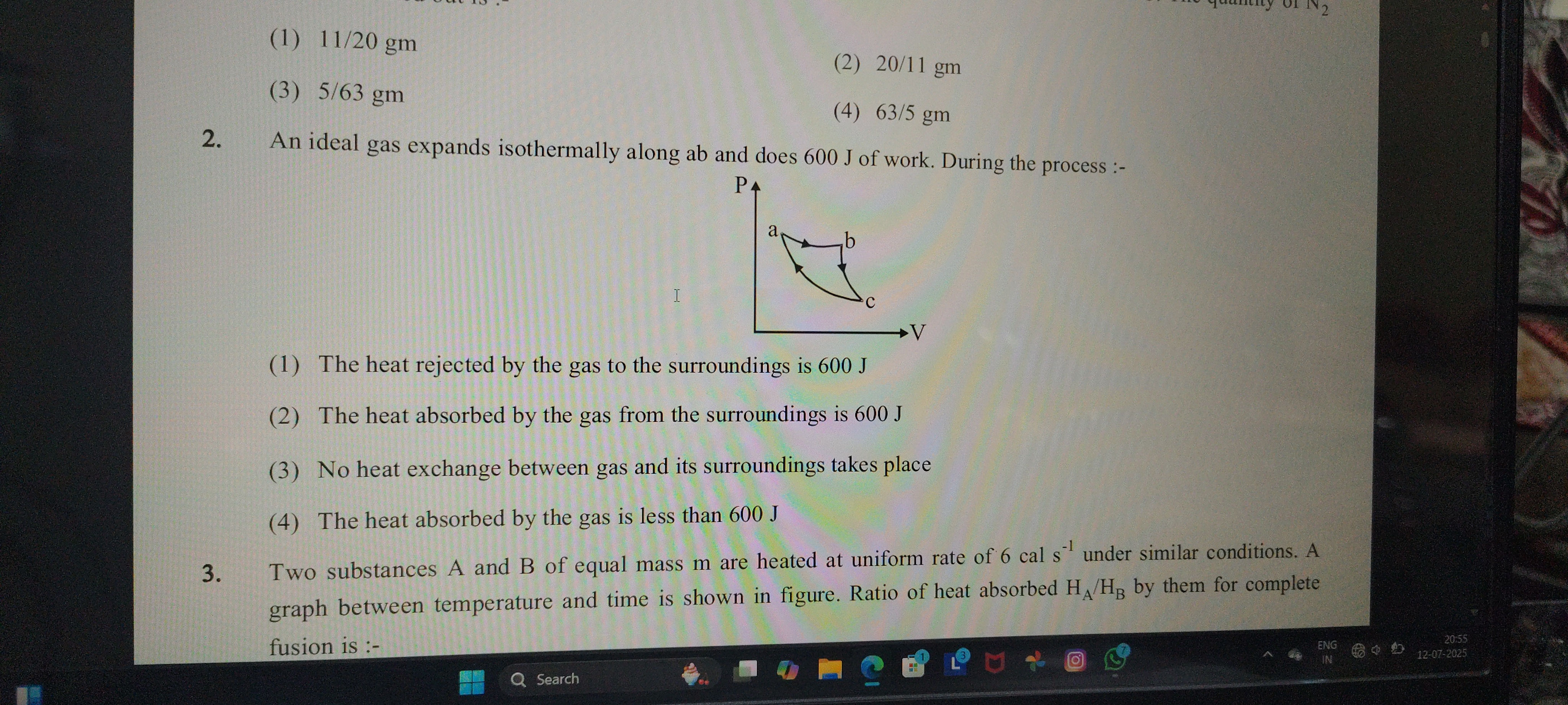
An ideal gas expands isothermally along ab and does 600 J of work. During the process :-
A
The heat rejected by the gas to the surroundings is 600 J
B
The heat absorbed by the gas from the surroundings is 600 J
C
No heat exchange between gas and its surroundings takes place
D
The heat absorbed by the gas is less than 600 J
Answer
The heat absorbed by the gas from the surroundings is 600 J
Explanation
Solution
For an ideal gas undergoing an isothermal process, the change in internal energy (ΔU) is zero because internal energy depends only on temperature, and temperature is constant. According to the First Law of Thermodynamics (ΔU=Q−W), if ΔU=0, then Q=W. Given that the gas does 600 J of work (W=+600 J), the heat absorbed by the gas (Q) must also be 600 J.
