Question
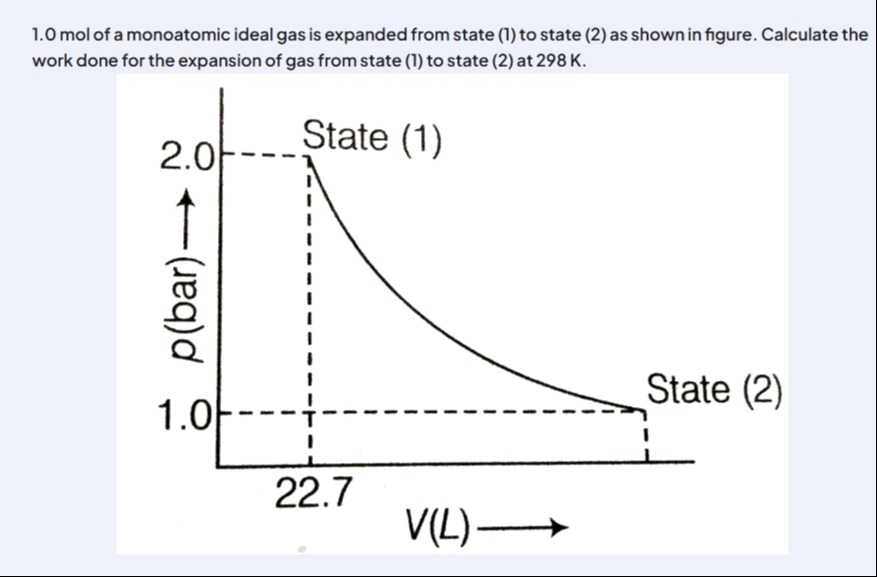
Question: 0 mol of a monoatomic ideal gas is expanded from state (1) to state (2) as shown in figure. Calculat...
0 mol of a monoatomic ideal gas is expanded from state (1) to state (2) as shown in figure. Calculate the work done for the expansion of gas from state (1) to state (2) at 298 K.
1720 J
Solution
The process depicted in the P-V diagram is an isothermal expansion, as indicated by the hyperbolic curve and the constant temperature of 298 K. For an ideal gas undergoing an isothermal process, the work done is calculated using the formula W=nRTln(P1/P2). Given n=1.0 mol, T=298 K, P1=2.0 bar, and P2=1.0 bar, and using the gas constant R=8.314 J/mol·K, we substitute these values into the formula to find the work done by the gas.
W=(1.0 mol)×(8.314 J/mol\cdotpK)×(298 K)×ln(1.0 bar2.0 bar) W=8.314×298×ln(2) W≈1717.94 J Rounding to three significant figures, the work done is approximately 1720 J.
