Question
Question: Indicate the IUPAC name of the compound. ...
Indicate the IUPAC name of the compound.
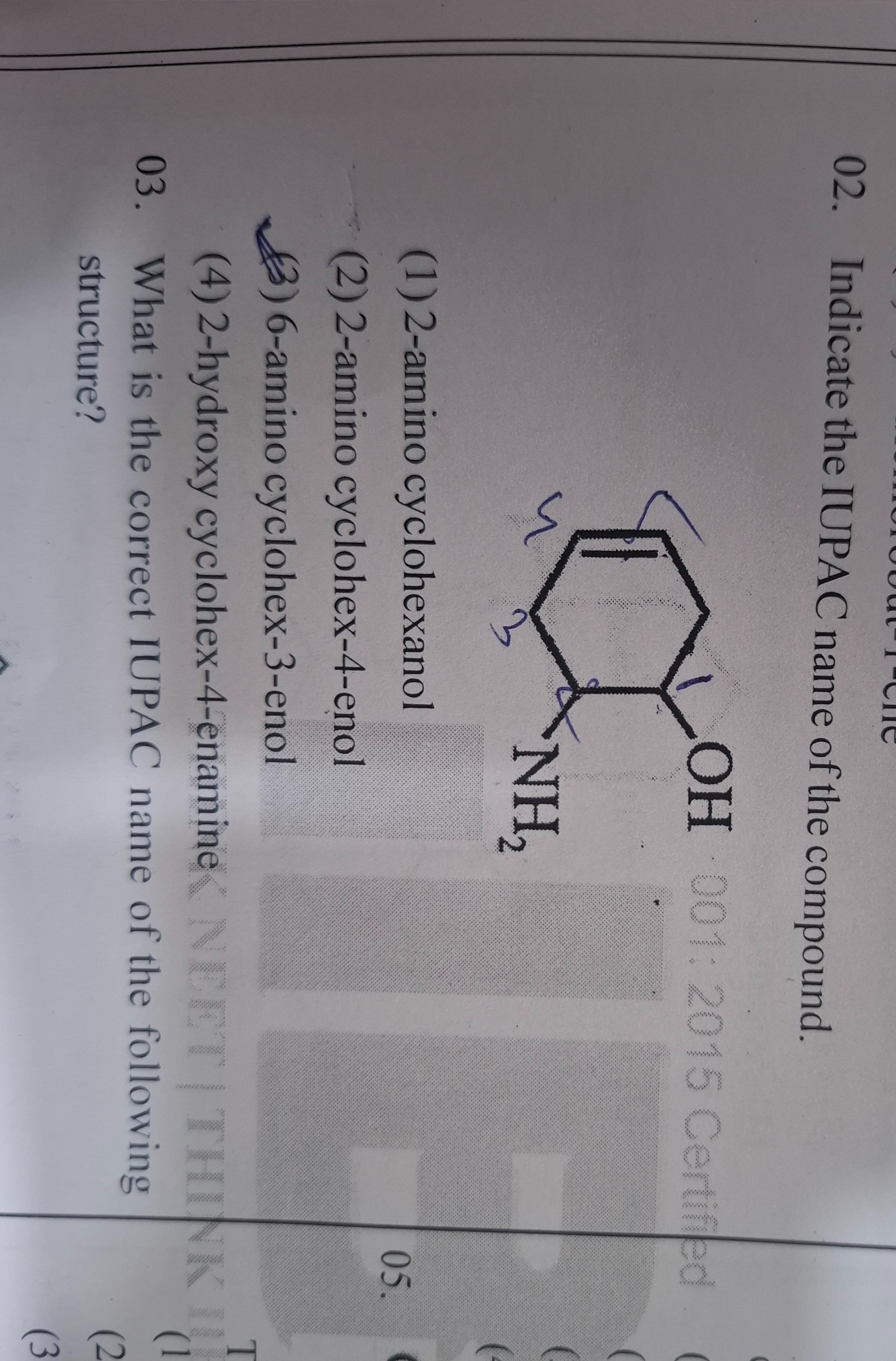
2-amino cyclohexanol
2-amino cyclohex-4-enol
6-amino cyclohex-3-enol
2-hydroxy cyclohex-4-enamine
3-amino-cyclohex-4-en-1-ol (Not in options, question is flawed)
Solution
To determine the IUPAC name of the compound, we follow these steps:
-
Identify the parent chain/ring: The compound has a six-membered ring with a double bond, so the parent ring is cyclohexene.
-
Identify functional groups and determine priority:
- -OH (hydroxyl group) is an alcohol, which has higher priority than an -NH2 (amino group) and an alkene (double bond).
- Therefore, the carbon atom bearing the -OH group will be assigned the lowest possible locant, which is C1. The suffix for the name will be "-ol".
-
Number the carbon atoms in the ring:
- Assign C1 to the carbon with the -OH group.
- Number the ring in a direction (clockwise or counter-clockwise) that gives the double bond the lowest possible locant. If both directions give the same lowest locant for the double bond, then choose the direction that gives the next substituent (-NH2) the lowest possible locant.
Let's analyze the given structure:
-
Starting from C1 (with -OH) and numbering clockwise:
- C1: -OH
- C2: (single bond)
- C3: -NH2
- C4: (part of double bond)
- C5: (other part of double bond)
- C6: (single bond)
- In this numbering, the -OH is at C1, the -NH2 is at C3, and the double bond is between C4 and C5 (starting at C4).
- Locants: 1(-OH), 3(-NH2), 4(-en).
- Name: 3-amino-cyclohex-4-en-1-ol.
-
Starting from C1 (with -OH) and numbering counter-clockwise:
- C1: -OH
- C6: (single bond)
- C5: (part of double bond)
- C4: (other part of double bond)
- C3: -NH2
- C2: (single bond)
- In this numbering, the -OH is at C1, the -NH2 is at C3, and the double bond is between C4 and C5 (starting at C4).
- Locants: 1(-OH), 3(-NH2), 4(-en).
- Name: 3-amino-cyclohex-4-en-1-ol.
Both numbering directions yield the same set of locants and thus the same name.
-
Construct the IUPAC name:
- Substituent: amino (at position 3)
- Parent ring: cyclohexene (double bond at position 4)
- Principal functional group: alcohol (at position 1)
- Combining these, the name is 3-amino-cyclohex-4-en-1-ol.
- Often, if the principal functional group is at C1, the "1" is omitted, so it could also be written as 3-amino-cyclohex-4-enol.
-
Compare with options:
- (1) 2-amino cyclohexanol: Incorrect, it lacks the double bond.
- (2) 2-amino cyclohex-4-enol: This would imply -OH at C1, -NH2 at C2, and double bond at C4. This does not match the given structure where -NH2 is at C3 relative to -OH at C1.
- (3) 6-amino cyclohex-3-enol: This would imply -OH at C1, -NH2 at C6, and double bond at C3. This does not match the given structure.
- (4) 2-hydroxy cyclohex-4-enamine: Incorrect. The -OH group has higher priority than -NH2, so the compound is an alcohol, not an amine.
The correct IUPAC name for the compound is 3-amino-cyclohex-4-en-1-ol, which is not among the options. Therefore, the question is flawed.
