Question
Question: Identify the product formed in the following reaction. $C_6H_5-CH_2-CH_3 \xrightarrow[(ii) \ H_3O^+...
Identify the product formed in the following reaction.
C6H5−CH2−CH3(i) alk.KMnO4(ii) H3O+ product
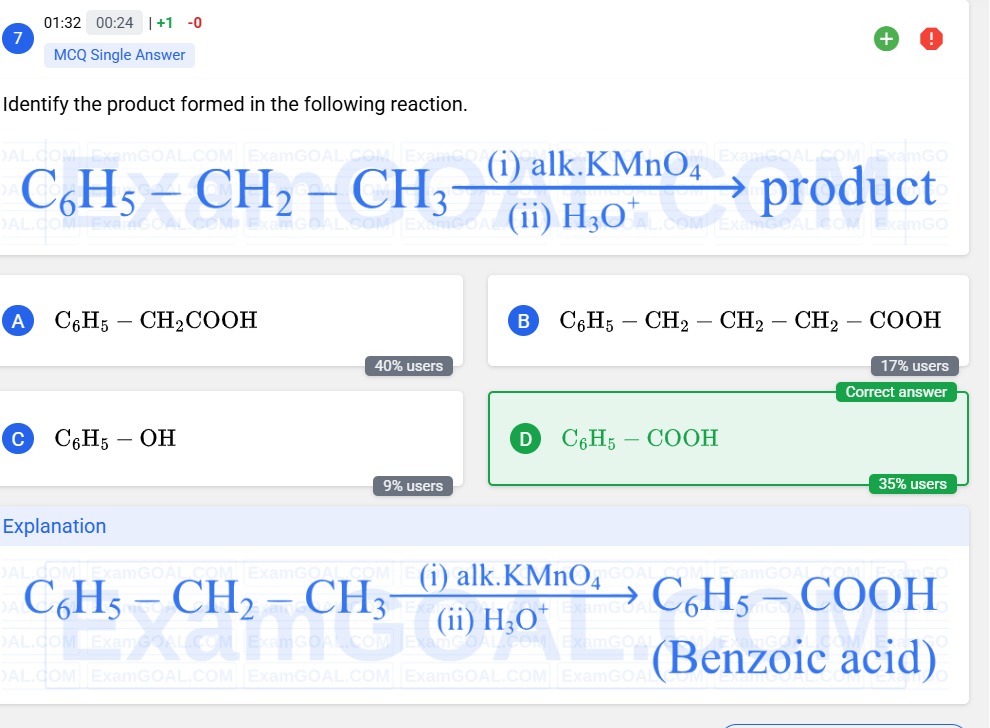
A
C6H5−CH2COOH
B
C6H5−CH2−CH2−CH2−COOH
C
C6H5−OH
D
C6H5−COOH
Answer
C6H5−COOH
Explanation
Solution
C6H5−CH2−CH3(i) alk.KMnO4(ii) H3O+C6H5−COOH
(Benzoic acid)
Alkaline KMnO4 oxidizes the benzylic side chain in ethylbenzene (which has at least one benzylic hydrogen) completely to a carboxyl group. Thus, after acid work-up with H3O+, the product is benzoic acid (C6H5–COOH).
