Question
Question: Which of the following statement is correct?...
Which of the following statement is correct?
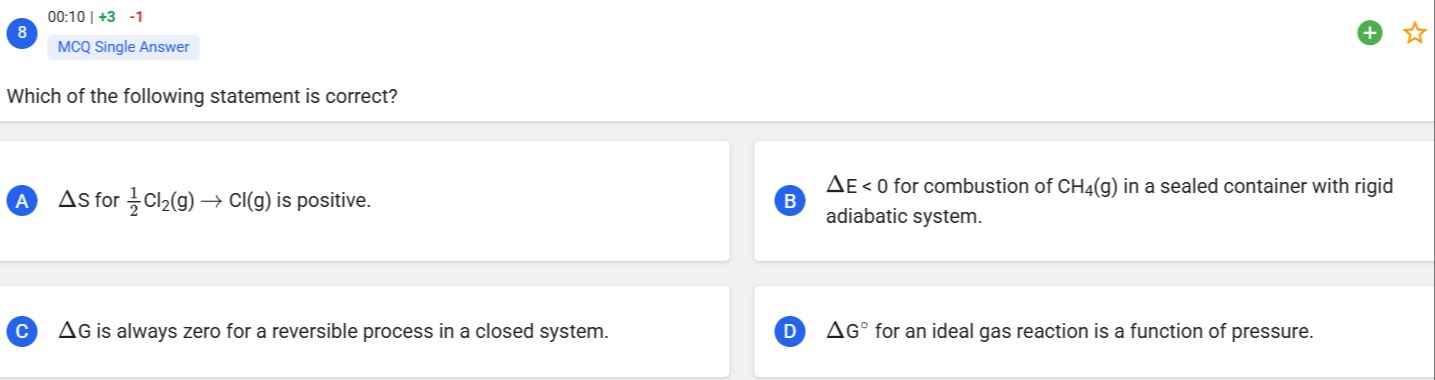
ΔS for 21Cl2(g)→Cl(g) is positive.
ΔE<0 for combustion of CH4(g) in a sealed container with rigid adiabatic system.
ΔG is always zero for a reversible process in a closed system.
ΔG∘ for an ideal gas reaction is a function of pressure.
ΔS for 21Cl2(g)→Cl(g) is positive.
Solution
The entropy change (ΔS) is positive for the reaction 21Cl2(g)→Cl(g) because one mole of gas is converted into two moles, increasing the disorder. Therefore, entropy increases.
In a rigid adiabatic system, ΔE=0 because no heat or work is exchanged with the surroundings.
ΔG=0 for a reversible process at constant temperature and pressure, but not always.
ΔG∘ is the standard Gibbs free energy change, which is a function of temperature, not pressure.
