Question
Question: The number of planes of symmetry in this compound is : ...
The number of planes of symmetry in this compound is :
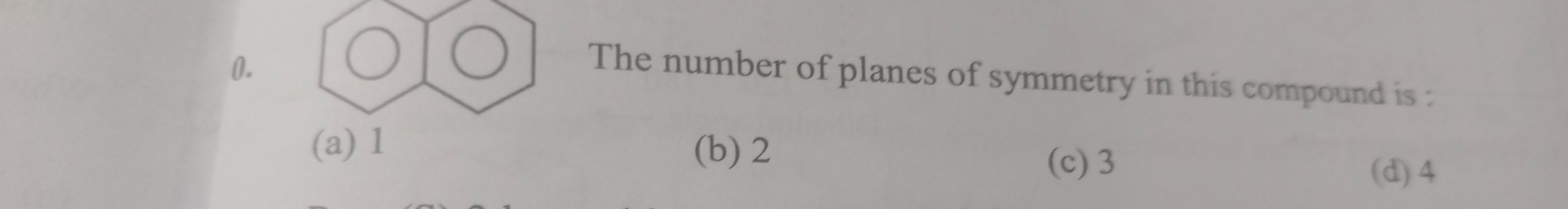
A
1
B
2
C
3
D
4
Answer
3
Explanation
Solution
The given compound is naphthalene, which has a D₂h symmetry. In this point group, there are three mutually perpendicular planes of symmetry:
-
One horizontal plane (the molecular plane)
-
Two vertical planes (one along the long axis and one perpendicular to it)
Thus, there are 3 planes of symmetry.
Naphthalene belongs to the D₂h group which has three mirror planes (one in the molecular plane and two vertical).
