Question
Question: \(0.1\)N solution of \[{\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\] is being...
0.1N solution of Na2CO3 is being titrated with 0.1N HCl, the best indicator to be used is:
A. potassium ferricyanide
B. phenolphthalein
C. methyl orange
D. litmus
Solution
When NaHCO3 + Na2CO3is titrated withHClin the presence of phenolphthalein indicator, the phenolphthalein indicator gives endpoint only at neutralization of 50%amount of the Na2CO3 whereas methyl orange indicator gives endpoint at complete neutralization of both of the compounds.
Complete step-by-step answer:
Potassium ferricyanide indicator is used for the titration where iron compound is used in the titration. Here, we are not dealing with the reaction of iron so, we cannot use potassium ferricyanide
It is given that Na2CO3 is being titrated with HCl. This titration reaction takes place in two steps.
Step-I: Na2CO3+HCl⇌NaHCO3 + NaCl
Step-II: NaHCO3+HCl⇌NaCl+H2CO3
Phenolphthalein is a basic indicator. It remains colourless below pH 8 and shows pink to dark red colour above pH 8.
So, if we use phenolphthalein indicator in Na2CO3and HCl it gives pink colour for step-I when NaHCO3 forms because NaHCO3 basic. So, as the NaHCO3 forms the pH of the solution increases and phenolphthalein gives pink colour. But in second step, H2CO3 forms which is an acid. As the H2CO3 forms the pH of the solution decreases. So, the phenolphthalein does not show colour for the second step.
So, we cannot use phenolphthalein indicator because we will get an end point of 50%titration only if we use phenolphthalein indicator.
Methyl orange is a weak base. Methyl orange indicator is used in the strong acid weak base titration.
The colour change of methyl orange depends upon the pH of the solution. In acidic medium means pH below 3.0 it shows red colour. In the range 3.0−4.5 pH, Methyl orange is of orange colour and in basic medium shows yellow colour.
So, methyl orange gives yellow colour at the first end point which confirms the completion of step-I and then the yellow colour changes to red colour at the second end point which confirms the completion of step-II.
So, the best indicator to be used for the titration of Na2CO3 with HCl is methyl orange.
Therefore, option (C) methyl orange is correct.
Note: Potassium ferricyanide indicator is used for the titration where iron metal undergoes oxidation. This indicator turns blue in presence of iron (II) so it indicates the oxidation of iron. The structure of methyl orange is as follows:
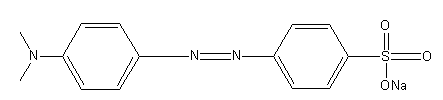
The change in methyl orange is represented as:
redHIn(aq)+H2O⇌H3O+(aq) + yellowIn−(aq). As the base is titrated with acid, initially in the basic medium methyl orange shows yellow colour. As the acid is added, concentration of acid increases, so the methyl orange remains in unionised form which is of red colour.
