Question
Question: Which of the following statement(s) is/are correct for decomposition of $NaHCO_3$?...
Which of the following statement(s) is/are correct for decomposition of NaHCO3?
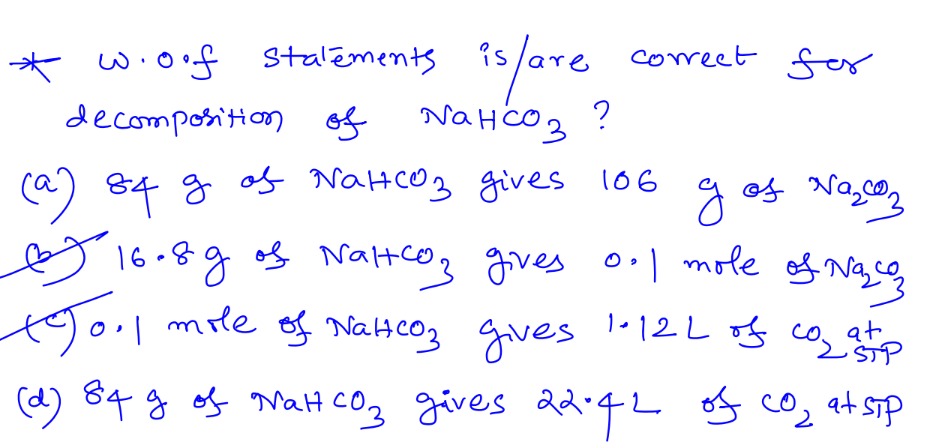
84 g of NaHCO3 gives 106 g of Na2CO3
16.8g of NaHCO3 gives 0.1 mole of Na2CO3
0.1 mole of NaHCO3 gives 1.12L of CO2 at STP
84 g of NaHCO3 gives 22.4L of CO2 at STP
Statements (b) and (c) are correct.
Solution
The decomposition of sodium bicarbonate (NaHCO3) follows the balanced chemical equation: 2NaHCO3(s)→Na2CO3(s)+H2O(g)+CO2(g)
Molar mass of NaHCO3=23(Na)+1(H)+12(C)+3×16(O)=84 g/mol. Molar mass of Na2CO3=2×23(Na)+12(C)+3×16(O)=106 g/mol. Molar volume of gas at STP = 22.4 L/mol.
(a) 84 g of NaHCO3 gives 106 g of Na2CO3 84 g of NaHCO3 is 1 mole. From the equation, 2 moles NaHCO3 produce 1 mole Na2CO3. So, 1 mole NaHCO3 produces 0.5 mole Na2CO3. Mass of 0.5 mole Na2CO3=0.5 mol×106 g/mol=53 g. This statement is incorrect.
(b) 16.8g of NaHCO3 gives 0.1 mole of Na2CO3 16.8 g of NaHCO3=16.8 g/84 g/mol=0.2 moles. 0.2 moles NaHCO3 produce (0.2/2)=0.1 mole of Na2CO3. This statement is correct.
(c) 0.1 mole of NaHCO3 gives 1.12L of CO2 at STP 0.1 mole NaHCO3 produce (0.1/2)=0.05 mole of CO2. Volume of CO2 at STP =0.05 mol×22.4 L/mol=1.12 L. This statement is correct.
(d) 84 g of NaHCO3 gives 22.4L of CO2 at STP 84 g of NaHCO3 is 1 mole. 1 mole NaHCO3 produces (1/2)=0.5 mole of CO2. Volume of CO2 at STP =0.5 mol×22.4 L/mol=11.2 L. This statement is incorrect.
