Question
Question: Calculate $W_{BC}$ for 1mol ideal gas...
Calculate WBC for 1mol ideal gas
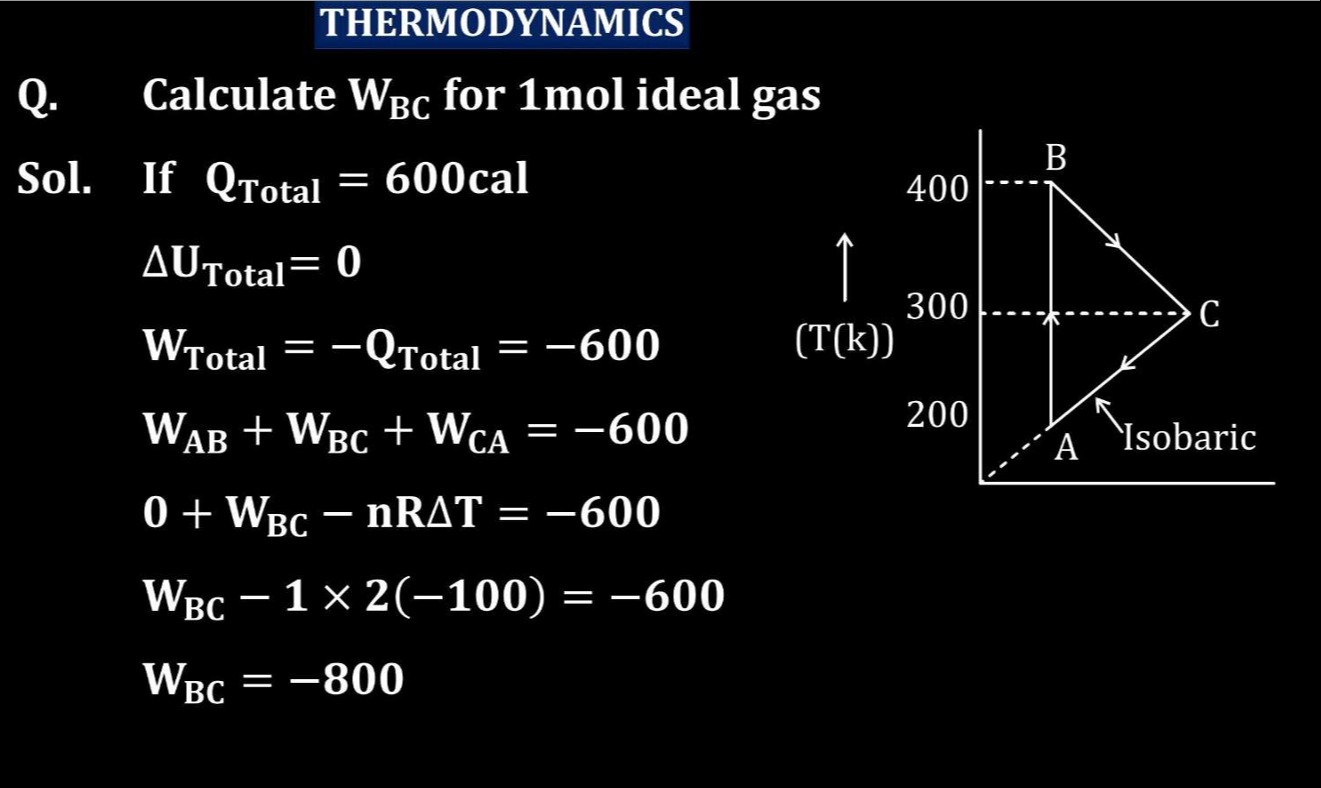
Answer
-800 cal
Explanation
Solution
To calculate WBC for 1 mol of an ideal gas:
- For cyclic process: ΔUTotal=0.
- First Law of Thermodynamics: ΔU=Q+W (where W is work done on the system).
- WTotal=−QTotal=−600 cal.
- WTotal=WAB+WBC+WCA.
- Process AB is isochoric, so WAB=0.
- Process CA is isobaric, so WCA=−nRΔTCA=−1×2×(200−300)=−1×2×(−100)=200 cal.
- Substitute values: −600=0+WBC+200.
- Solve for WBC: WBC=−600−200=−800 cal.
