Question
Question: The given pair are : and ...
The given pair are :
and
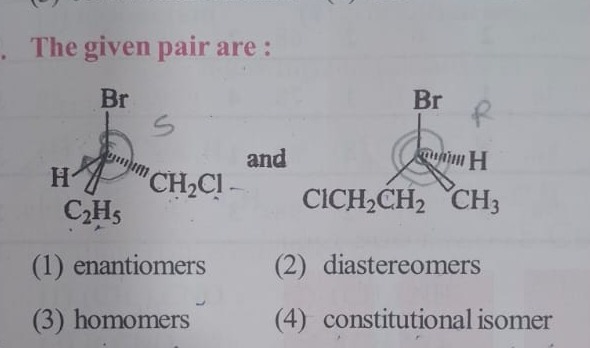
A
enantiomers
B
diastereomers
C
homomers
D
constitutional isomer
Answer
constitutional isomer
Explanation
Solution
Explanation:
- In the first molecule, the central carbon is bonded to Br, H, C₂H₅, and CH₂Cl.
- In the second molecule, the central carbon is bonded to Br, H, CH₃, and CH₂CH₂Cl.
- Although both molecules have a chiral center, the connectivity of the substituents differs (chlorine is attached to different carbon chains in each case).
- This difference in connectivity classifies the pair as constitutional isomers.
Answer:
Option (4) constitutional isomers
