Question
Question: Sample of $C_2H_6$ has same mass as $10^7$ molecules of $CH_y$. Howmany no. of molecules does the sa...
Sample of C2H6 has same mass as 107 molecules of CHy. Howmany no. of molecules does the sample contain.
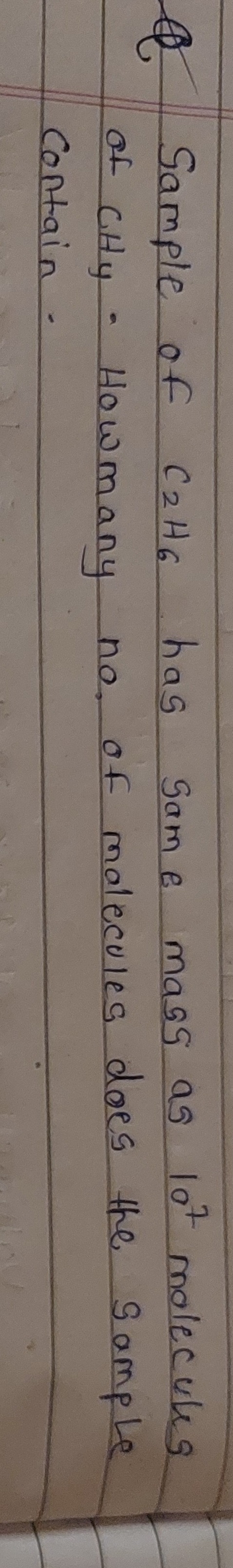
Answer
30107(12+y) molecules
Explanation
Solution
Let the number of molecules of C2H6 in the sample be N. The mass of one molecule of any compound is proportional to its molecular mass (in atomic mass units). Thus,
Mass of one C2H6Mass of one CHy∝∝2(12)+6(1)=3012+ySince the mass of the C2H6 sample equals the mass of 107 molecules of CHy:
N×30=107×(12+y)Solving for N:
N=30107×(12+y)Explanation (Minimal):
- Calculate molecular mass: C2H6=30 and CHy=12+y.
- Using the equality of masses: N⋅30=107(12+y).
- Solve: N=30107(12+y).
