Question
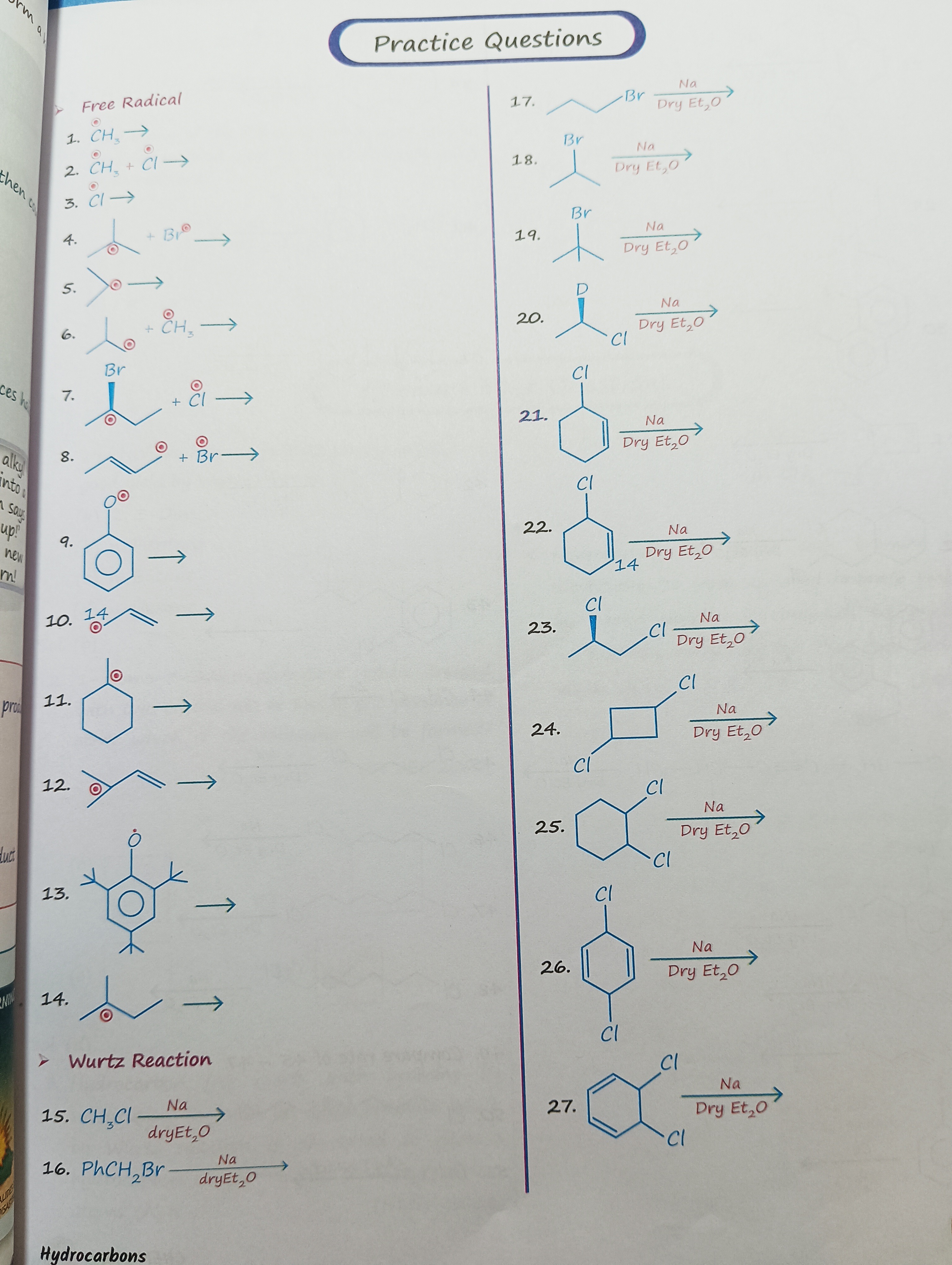
Question: Predict the products for the following reactions: ➤ Free Radical 1. $CH_3 \cdot$ → 2. $CH_3 \cdot...
Predict the products for the following reactions:
➤ Free Radical
- CH3⋅ →
- CH3⋅+Cl⋅ →
- Cl⋅ →
- Isopropyl radical + Br⋅ →
- Isopropyl radical →
- Isopropyl radical + CH3⋅ →
- Isopropyl radical + Cl⋅ →
- Allyl radical + Br⋅ →
- Phenoxy radical →
- Allyl radical →
- Cyclohexyl radical →
- CH2=CH−CH(CH3)⋅ →
- Substituted benzyl radical →
- Isopropyl radical →
➤ Wurtz Reaction 15. CH3CldryEt2ONa → 16. PhCH2BrdryEt2ONa → 17. Propyl bromide Dry Et2ONa 18. Isopropyl bromide Dry Et2ONa 19. tert-Butyl bromide Dry Et2ONa 20. Chlorobenzene Dry Et2ONa 21. 1-chlorocyclohexene Dry Et2ONa 22. 1-chlorocyclohexane Dry Et2ONa 23. 1,2-dichlorocyclohexane Dry Et2ONa 24. 1,4-dichlorocyclohexane Dry Et2ONa 25. 1,4-dichlorocyclohexane Dry Et2ONa 26. 1,2-dichlorobenzene Dry Et2ONa 27. Benzene Dry Et2ONa
- Ethane (CH3−CH3)
- Methyl chloride (CH3Cl)
- Chlorine (Cl2)
- Isopropyl bromide (CH(CH3)2Br)
- 2,3-dimethylbutane (CH(CH3)2−CH(CH3)2)
- 2-methylbutane (CH(CH3)2−CH3)
- Isopropyl chloride (CH(CH3)2Cl)
- Allyl bromide (CH2=CH−CH2Br)
- Dimerization (e.g., Diphenyl peroxide Ph−O−O−Ph, or C-C coupled products like 4,4'-diphenoxy)
- 1,5-hexadiene (CH2=CH−CH2−CH2−CH=CH2)
- Bicyclohexyl
- Dimer of CH2=CH−CH(CH3)⋅
- Dimer of the substituted benzyl radical
- 2,3-dimethylbutane (CH(CH3)2−CH(CH3)2)
- Ethane (CH3−CH3)
- 1,2-diphenylethane (PhCH2−CH2Ph)
- n-Hexane (CH3CH2CH2−CH2CH2CH3)
- 2,3-dimethylbutane (CH(CH3)2−CH(CH3)2)
- 2,2,3,3-tetramethylbutane ((CH3)3C−C(CH3)3)
- Biphenyl
- Dimer of 1-cyclohexenyl radical
- Bicyclohexyl
- Bicyclo[4.1.0]heptane
- Dimer of cyclohexyl derivative (intermolecular coupling)
- Dimer of cyclohexyl derivative (intermolecular coupling)
- Biphenyl
- No reaction
Solution
The questions cover two main topics: Free Radical reactions and Wurtz Reaction.
Free Radical Reactions: These questions involve the formation, reaction, or dimerization of free radicals. A radical is an atom or molecule with an unpaired electron, indicated by a dot (⋅).
- Radical combination/termination: When two radicals combine, they form a stable molecule. For example, CH3⋅+Cl⋅→CH3Cl.
- Radical dimerization: When a single radical species is present, it often dimerizes to form a stable molecule by forming a new bond between two radical centers. For example, 2CH3⋅→CH3−CH3.
- Resonance stabilization: Some radicals, like the phenoxy radical, are resonance stabilized, meaning the unpaired electron is delocalized. Dimerization can occur through coupling at different positions.
Wurtz Reaction: This reaction involves the coupling of two alkyl halide molecules using sodium metal in a dry ethereal solvent (like dry Et2O) to form a new alkane. The general reaction is: 2R−X+2Nadry Et2OR−R+2NaX
- The reaction works best with primary and secondary alkyl halides.
- Tertiary alkyl halides are prone to elimination reactions.
- Aryl halides typically do not undergo Wurtz reaction; they react in Wurtz-Fittig reactions or can form biphenyls under certain conditions.
- Vicinal dihalides can undergo intramolecular cyclization to form cyclic compounds if a stable ring can be formed.
