Question
Question: (-m-Group) = sre meta - director. # Meta bypass. When 2-groups, one is + m -Group & the another is...
(-m-Group) = sre meta - director.
Meta bypass.
When 2-groups, one is + m -Group & the another is - m group are converted to each other (at m-bali) with the benzenle ring, then they are in the resonance with the ring but not in resonance with each other.
eg:-
-ve charge bypass - Cho ve "
- on.
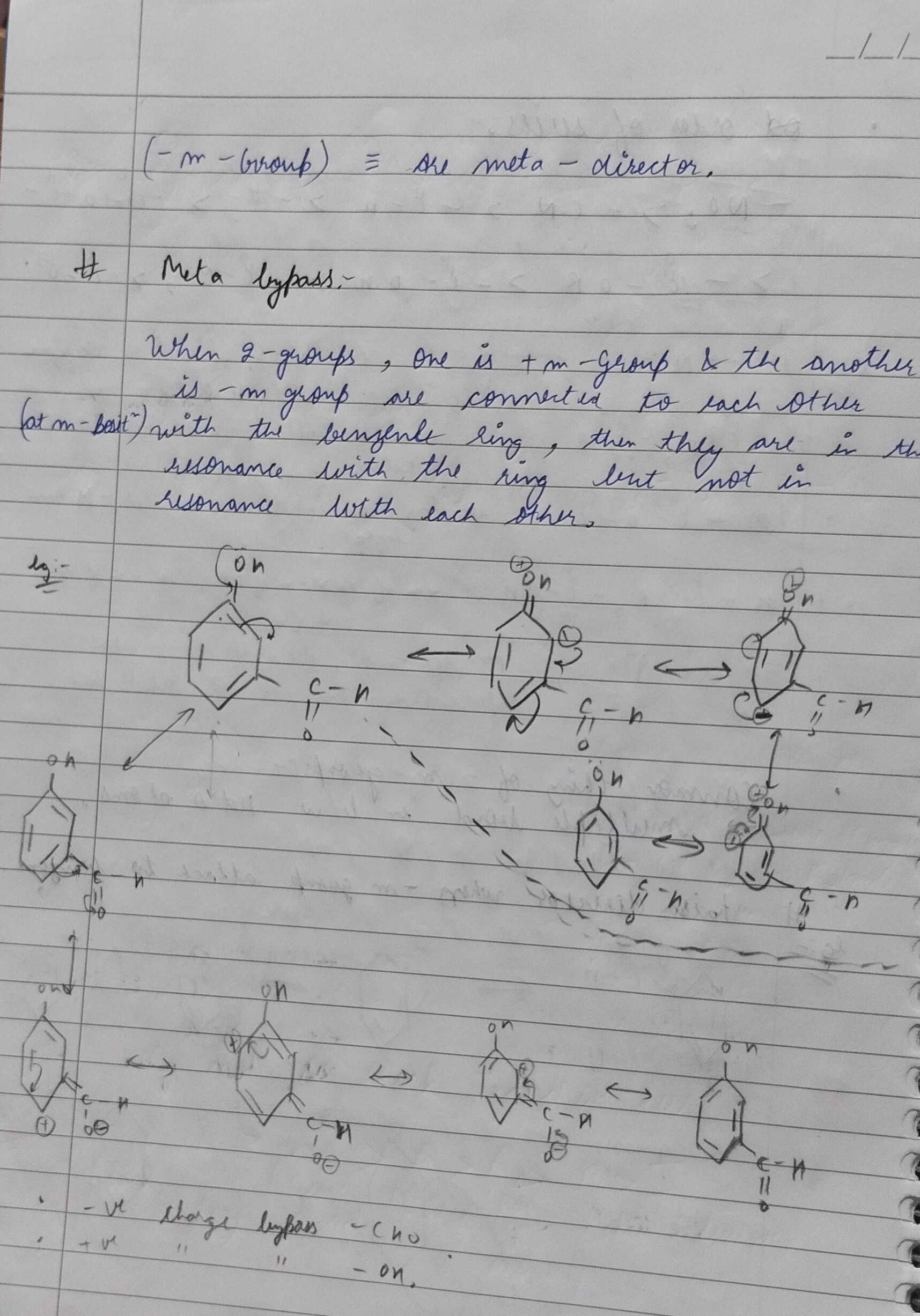
The provided text and diagrams explain the concept of "Meta bypass" in organic chemistry, specifically concerning the resonance effects of substituents on a benzene ring. It states that when a +M group and a -M group are meta to each other on a benzene ring, they resonate with the ring but not directly with each other. This leads to independent (or less interdependent) mesomeric influences on the ring's electron density. The example of 3-hydroxybenzaldehyde illustrates how the -OH group (a +M group) donates electrons to its ortho/para positions, and the -CHO group (a -M group) withdraws electrons from its ortho/para positions, without direct resonance interaction between the two groups themselves.
Solution
The provided notes describe a concept called "Meta bypass" in the context of substituted benzene rings, specifically when two groups with opposing mesomeric effects (+M and -M) are present at meta positions to each other.
Explanation of the Solution:
-
General Rule for -M Groups: A group exhibiting a negative mesomeric effect (-M group) withdraws electron density from the benzene ring via resonance. This deactivates the ortho and para positions and directs incoming electrophiles to the meta position. Hence, -M groups are generally meta-directors.
-
Meta Bypass Condition: The "Meta bypass" phenomenon occurs when a benzene ring is disubstituted with two groups, one being a +M (electron-donating by resonance) group and the other a -M (electron-withdrawing by resonance) group, and these two groups are positioned meta to each other.
-
Mechanism of Meta Bypass: In this specific meta arrangement, the two groups are in resonance with the benzene ring, meaning they can either donate or withdraw electrons from the ring through conjugation. However, crucially, they are not in direct resonance with each other. This implies that their mesomeric effects on the ring's electron density are largely independent.
-
Example: 3-Hydroxybenzaldehyde (m-hydroxybenzaldehyde) Let's consider 3-hydroxybenzaldehyde, where the -OH (hydroxyl) group is a +M group and the -CHO (aldehyde) group is a -M group, and they are meta to each other.
-
Resonance involving -OH (+M Group): The lone pair electrons on the oxygen of the -OH group are donated into the benzene ring. This creates a negative charge at the ortho and para positions relative to the -OH group (positions 2, 4, and 6 if -OH is at 1). The oxygen atom acquires a positive charge.
The resonance structures illustrate this:
\begin{center} \includegraphics[width=0.8\textwidth]{resonance_OH_part1.png} \end{center}(Representing the first three structures from the top row of the diagram showing -OH donating electrons)
-
Resonance involving -CHO (-M Group): The pi electrons from the benzene ring are withdrawn towards the highly electronegative oxygen of the carbonyl group in -CHO. This creates a positive charge (electron deficiency) at the ortho and para positions relative to the -CHO group (positions 2, 4, and 6 if -CHO is at 3). The oxygen atom of the carbonyl group acquires a negative charge.
The resonance structures illustrate this:
\begin{center} \includegraphics[width=0.8\textwidth]{resonance_CHO_part2.png} \end{center}(Representing the first three structures from the bottom row of the diagram showing -CHO withdrawing electrons)
-
-
Significance of "Bypass": The phrases "-ve charge bypass - Cho" and "+ve charge bypass - OH" highlight that because the +M and -M groups are meta to each other, the electron density donated by the +M group does not directly flow into the -M group, and the electron deficiency created by the -M group is not directly compensated by the +M group. Their resonance interactions are primarily with the benzene ring itself, not directly between each other. This means that the positions meta to one group are not directly involved in the resonance effects of the other group, offering a "bypass" from the direct conjugation path.
In summary, "Meta bypass" describes the scenario where meta-disposed +M and -M groups on a benzene ring exert their mesomeric effects largely independently on the ring, without direct resonance interaction between themselves.
