Question
Question: For following reaction, relation between $\Delta H$ and $\Delta U$ is $SO_{2(g)} + O_{2(g)} \righta...
For following reaction, relation between ΔH and ΔU is
SO2(g)+O2(g)→2SO3(g)
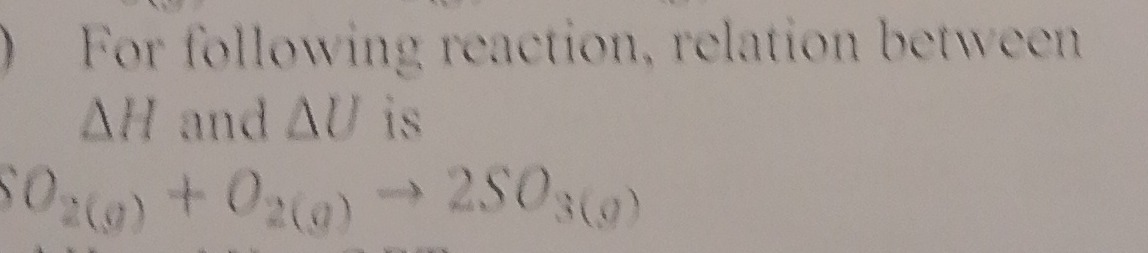
Answer
ΔH = ΔU
Explanation
Solution
For a reaction at constant pressure, the relation is given by
ΔH=ΔU+Δ(PV)=ΔU+ΔnRT.For the reaction
SO2(g)+O2(g)→2SO3(g),the initial moles of gas are 1+1=2 and the final moles are 2. Thus,
Δn=2−2=0.Therefore,
ΔH=ΔU+0⋅RT=ΔU.ΔH = ΔU + ΔnRT; here Δn = 0, so ΔH = ΔU.
