Question
Question: Consider the following alkenes with regard to their stability...
Consider the following alkenes with regard to their stability
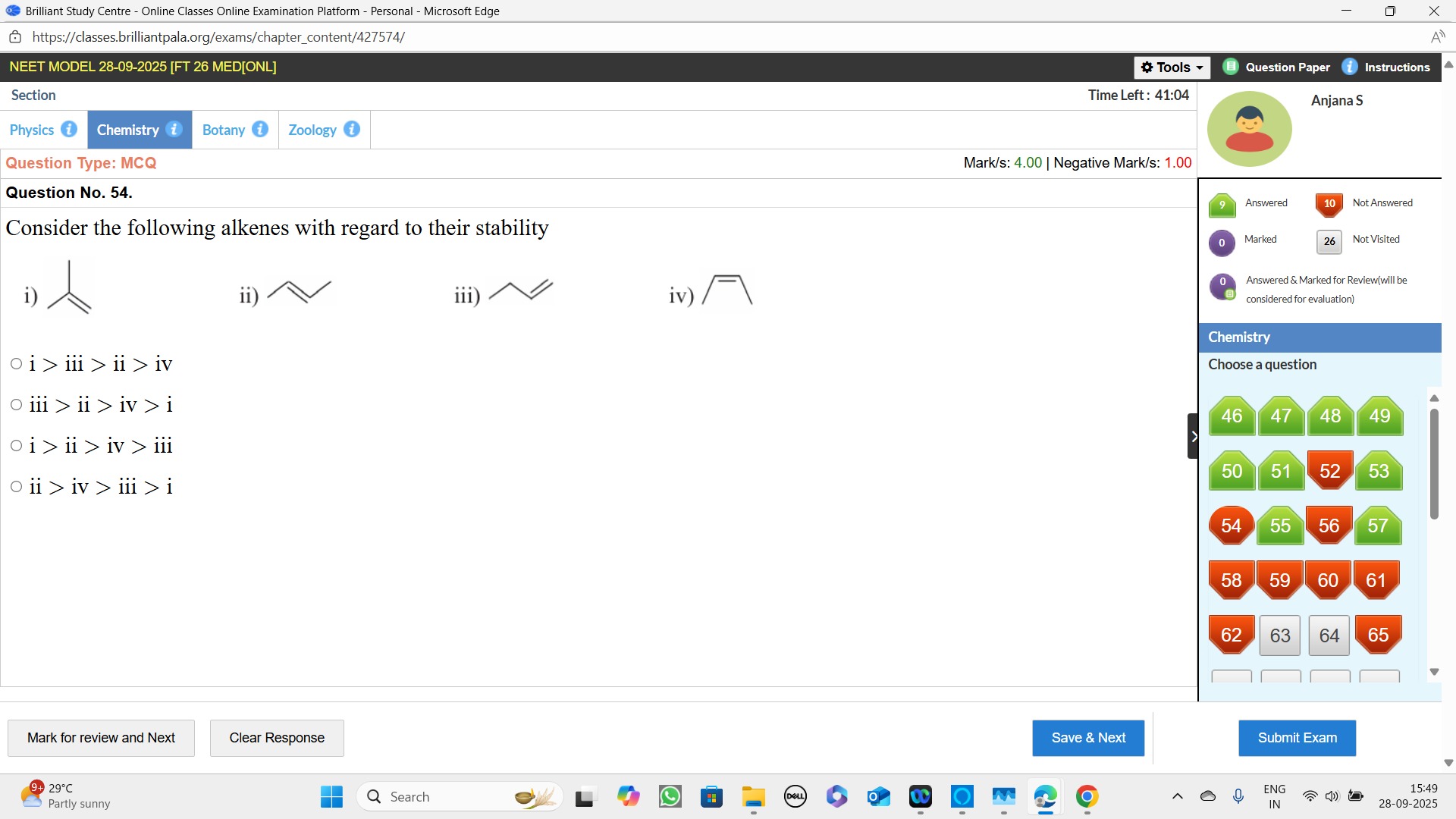
i > iii > ii > iv
iii > ii > iv > i
i > ii > iv > iii
ii > iv > iii > i
i > ii > iv > iii
Solution
The stability of alkenes is primarily determined by the degree of substitution of the double bond carbons and the number of alpha-hydrogens, which indicate hyperconjugation. Heat of hydrogenation is a quantitative measure of alkene stability; a less exothermic hydrogenation (less negative enthalpy change) indicates a more stable alkene.
- Alkene i: 2-methylpropene (CH2=C(CH3)2). This is a disubstituted alkene with 6 alpha-hydrogens.
- Alkene ii: But-2-ene (CH3-CH=CH-CH3). This is a disubstituted alkene with 6 alpha-hydrogens.
- Alkene iii: But-1-ene (CH3-CH2-CH=CH2). This is a monosubstituted alkene with 3 alpha-hydrogens.
- Alkene iv: Cyclohexene. This is a cyclic disubstituted alkene with 4 alpha-hydrogens.
Based on heats of hydrogenation (experimental values):
- Alkene i: ΔHhydrog≈−112.5 kJ/mol
- Alkene ii: ΔHhydrog≈−115.2 kJ/mol
- Alkene iv: ΔHhydrog≈−119.5 kJ/mol
- Alkene iii: ΔHhydrog≈−126 kJ/mol
A less exothermic heat of hydrogenation indicates a more stable alkene. Therefore, the stability order from most stable to least stable is: i (-112.5 kJ/mol) > ii (-115.2 kJ/mol) > iv (-119.5 kJ/mol) > iii (-126 kJ/mol).
This order is i > ii > iv > iii.
