Question
Question: For the reaction, $Fe_3N(s) + \frac{3}{2}H_2(g) \rightleftharpoons 3Fe(s) + NH_3(g)$...
For the reaction, Fe3N(s)+23H2(g)⇌3Fe(s)+NH3(g)
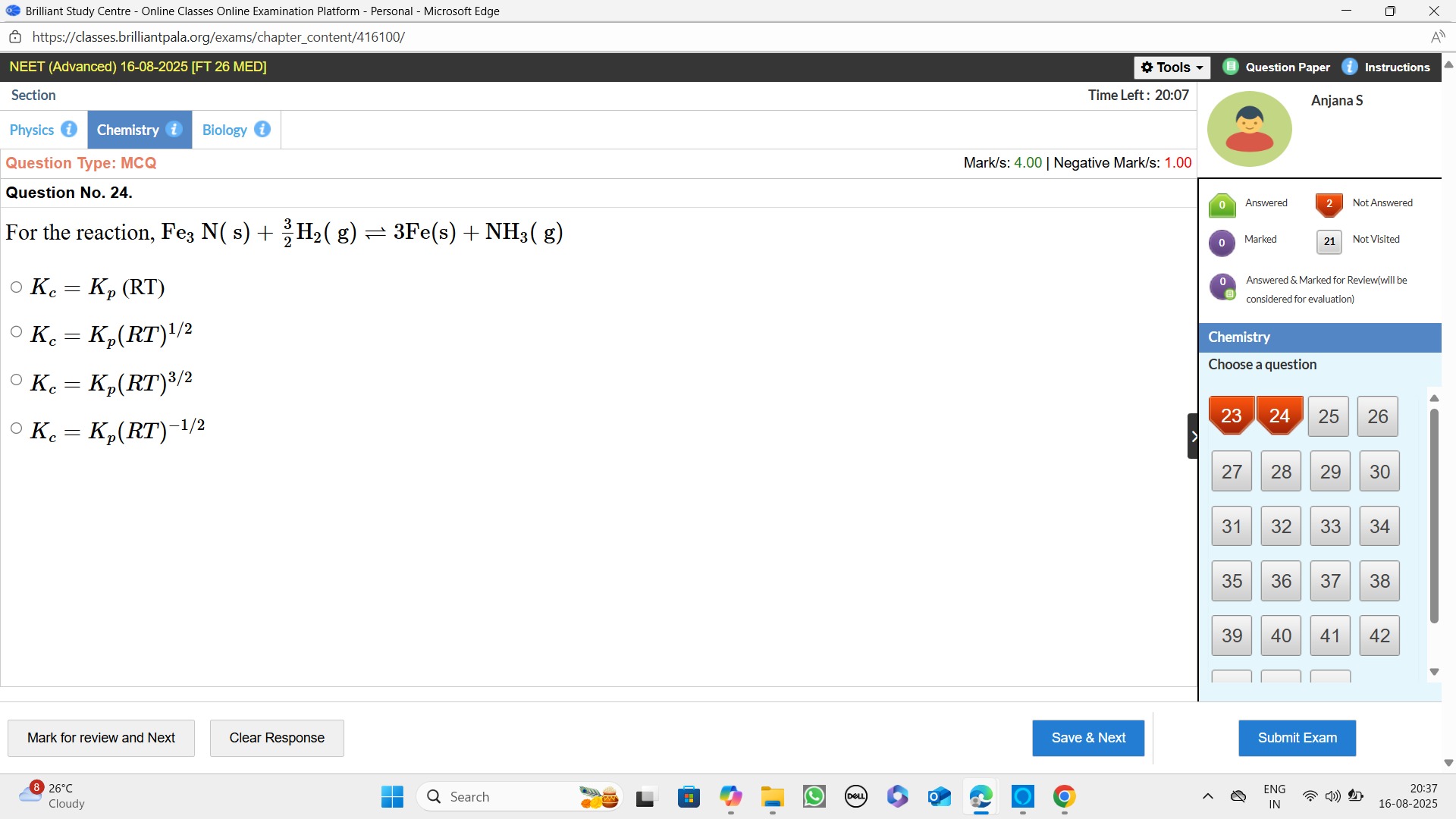
Kc=Kp(RT)
Kc=Kp(RT)1/2
Kc=Kp(RT)3/2
Kc=Kp(RT)−1/2
Kc=Kp(RT)1/2
Solution
The relationship between Kp and Kc for a reaction is given by the equation:
Kp=Kc(RT)Δng
Where:
Kp = Equilibrium constant in terms of partial pressures
Kc = Equilibrium constant in terms of molar concentrations
R = Ideal gas constant
T = Absolute temperature in Kelvin
Δng = (Sum of stoichiometric coefficients of gaseous products) - (Sum of stoichiometric coefficients of gaseous reactants)
The given reaction is:
Fe3N(s)+23H2(g)⇌3Fe(s)+NH3(g)
To calculate Δng, we only consider the gaseous species.
Moles of gaseous products (np,g):
For NH3(g), the stoichiometric coefficient is 1.
So, np,g=1.
Moles of gaseous reactants (nr,g):
For H2(g), the stoichiometric coefficient is 23.
So, nr,g=23.
Now, calculate Δng:
Δng=np,g−nr,g
Δng=1−23
Δng=22−23
Δng=−21
Substitute the value of Δng into the relationship between Kp and Kc:
Kp=Kc(RT)−1/2
The question asks for the relationship expressed in terms of Kc. Rearrange the equation:
Kc=(RT)−1/2Kp
Kc=Kp(RT)1/2
Comparing this with the given options, the second option matches our derived relationship.
