Question
Question: $S_2O_8^{2-}$(aq) oxidises $Mn^{2+}$(aq) to $MnO_4^-$ (aq) in acidic medium. The number of moles of ...
S2O82−(aq) oxidises Mn2+(aq) to MnO4− (aq) in acidic medium. The number of moles of S2O82− required to oxidise one mole Mn2+ to MnO4− is
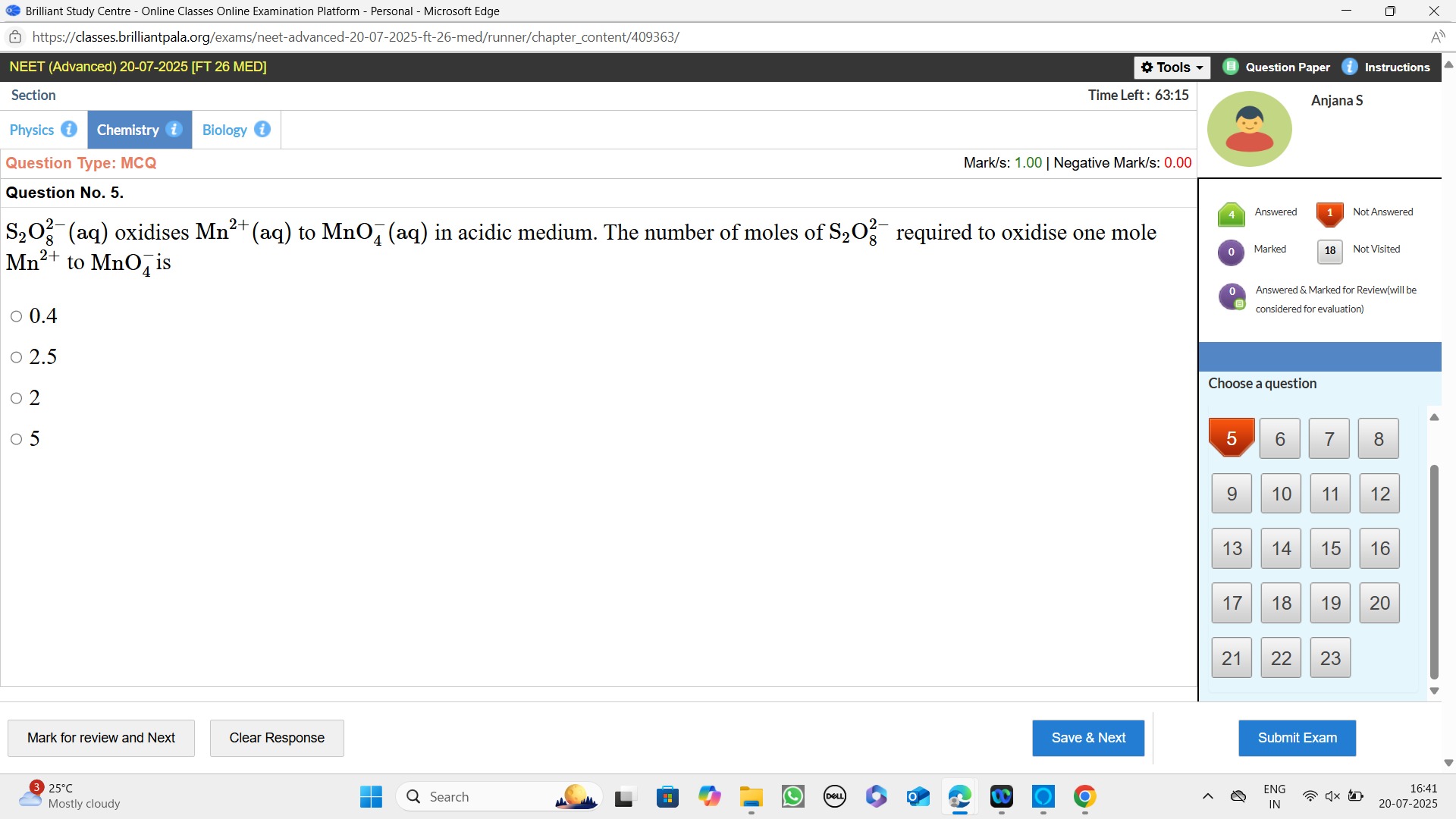
0.4
2.5
2
5
2.5
Solution
The problem asks for the number of moles of S2O82− required to oxidize one mole of Mn2+ to MnO4− in an acidic medium. We can solve this by balancing the redox reaction or by using the concept of n-factors (equivalents).
Method 1: Balancing the Redox Reaction
-
Identify the oxidation and reduction half-reactions:
- Oxidation: Mn2+→MnO4−
- Reduction: S2O82−→SO42−
-
Balance the oxidation half-reaction: Mn2+ has an oxidation state of +2. In MnO4−, Mn has an oxidation state of +7 (since x+4(−2)=−1⇒x=+7). The change in oxidation state for Mn is 7−2=5. So, 5 electrons are lost. Balance oxygen atoms by adding H2O and hydrogen atoms by adding H+ (acidic medium): Mn2++4H2O→MnO4−+8H++5e−
-
Balance the reduction half-reaction: In S2O82− (peroxodisulfate ion), the average oxidation state of sulfur is +7 (since 2x+8(−2)=−2⇒2x=14⇒x=+7). In SO42−, sulfur has an oxidation state of +6 (since y+4(−2)=−2⇒y=+6). The change in oxidation state per sulfur atom is 7−6=1. Since there are two sulfur atoms in S2O82− that get reduced to 2SO42−, a total of 2×1=2 electrons are gained. Balance the sulfur atoms first: S2O82−→2SO42− Now, add electrons to balance the charge: S2O82−+2e−→2SO42−
-
Equalize the number of electrons transferred: The oxidation half-reaction involves 5 electrons, and the reduction half-reaction involves 2 electrons. To balance the electrons, multiply the oxidation half-reaction by 2 and the reduction half-reaction by 5.
- 2×(Mn2++4H2O→MnO4−+8H++5e−)
2Mn2++8H2O→2MnO4−+16H++10e− * 5×(S2O82−+2e−→2SO42−)
5S2O82−+10e−→10SO42−
- Add the balanced half-reactions:
2Mn2++8H2O+5S2O82−+10e−→2MnO4−+16H++10e−+10SO42− Cancel out the electrons:
2Mn2+(aq)+5S2O82−(aq)+8H2O(l)→2MnO4−(aq)+10SO42−(aq)+16H+(aq)
- Determine the mole ratio: From the balanced equation, 2 moles of Mn2+ react with 5 moles of S2O82−. Therefore, for 1 mole of Mn2+, the moles of S2O82− required will be: Moles of S2O82−=2 moles Mn2+5 moles S2O82−×1 mole Mn2+=2.5 moles S2O82−
Method 2: Using n-factors (Equivalents Concept)
The number of equivalents of oxidant must be equal to the number of equivalents of reductant. Number of equivalents = number of moles × n-factor
- Calculate the n-factor for Mn2+:
Mn2+→MnO4− Oxidation state change: +2 to +7. Number of electrons lost = 5. So, n-factor (nMn2+) = 5.
- Calculate the n-factor for S2O82−:
S2O82−→2SO42− This reaction involves the gain of 2 electrons per mole of S2O82−. So, n-factor (nS2O82−) = 2.
- Apply the equivalence principle: Moles of S2O82− ×nS2O82− = Moles of Mn2+ ×nMn2+ Let 'x' be the moles of S2O82− required.
x×2=1×5
2x=5
x=25=2.5
Both methods yield the same result.
The balanced redox reaction is:
2Mn2+(aq)+5S2O82−(aq)+8H2O(l)→2MnO4−(aq)+10SO42−(aq)+16H+(aq)
From the stoichiometry, 2 moles of Mn2+ are oxidized by 5 moles of S2O82−. Therefore, 1 mole of Mn2+ requires 25=2.5 moles of S2O82−.
