Question
Question: A sample of $CaCO_3$ has 193000 C of charge in magniltide due to its anions only. Find the no. of mo...
A sample of CaCO3 has 193000 C of charge in magniltide due to its anions only. Find the no. of moles of CaCO3 in the sample. (mag. of charge on 1 mole e− = 96500 C)
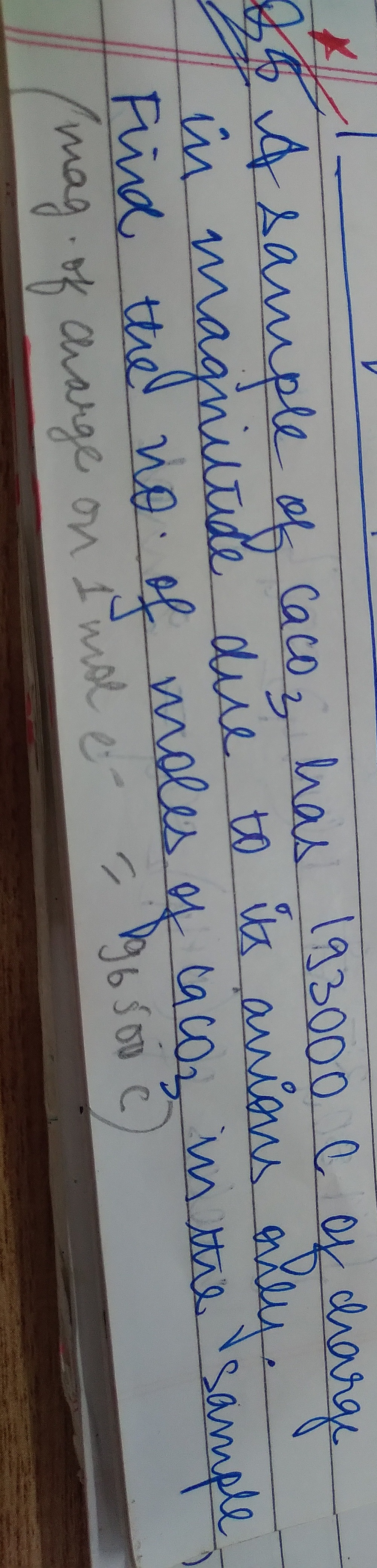
1 mole
2 moles
0.5 moles
0.25 moles
1 mole
Solution
The anion in CaCO3 is the carbonate ion, CO32−. The magnitude of the charge on the carbonate ion is 2. The magnitude of charge on 1 mole of electrons is given as 96500 C (Faraday's constant, F). Therefore, the magnitude of charge on 1 mole of CO32− anions is 2×96500 C = 193000 C.
The total charge due to anions in the sample is given as 193000 C. Let n be the number of moles of CaCO3. Since one mole of CaCO3 contains one mole of CO32− ions, 'n' moles of CaCO3 contain 'n' moles of CO32− ions.
The total charge is calculated as: Total Charge = (Number of moles of anions) × (Charge per mole of anion) 193000 C=n×(193000 C/mol)
Solving for n: n=193000 C/mol193000 C n=1 mole
Thus, the number of moles of CaCO3 in the sample is 1 mole.
