Question
Question: $\longrightarrow$ ...
⟶
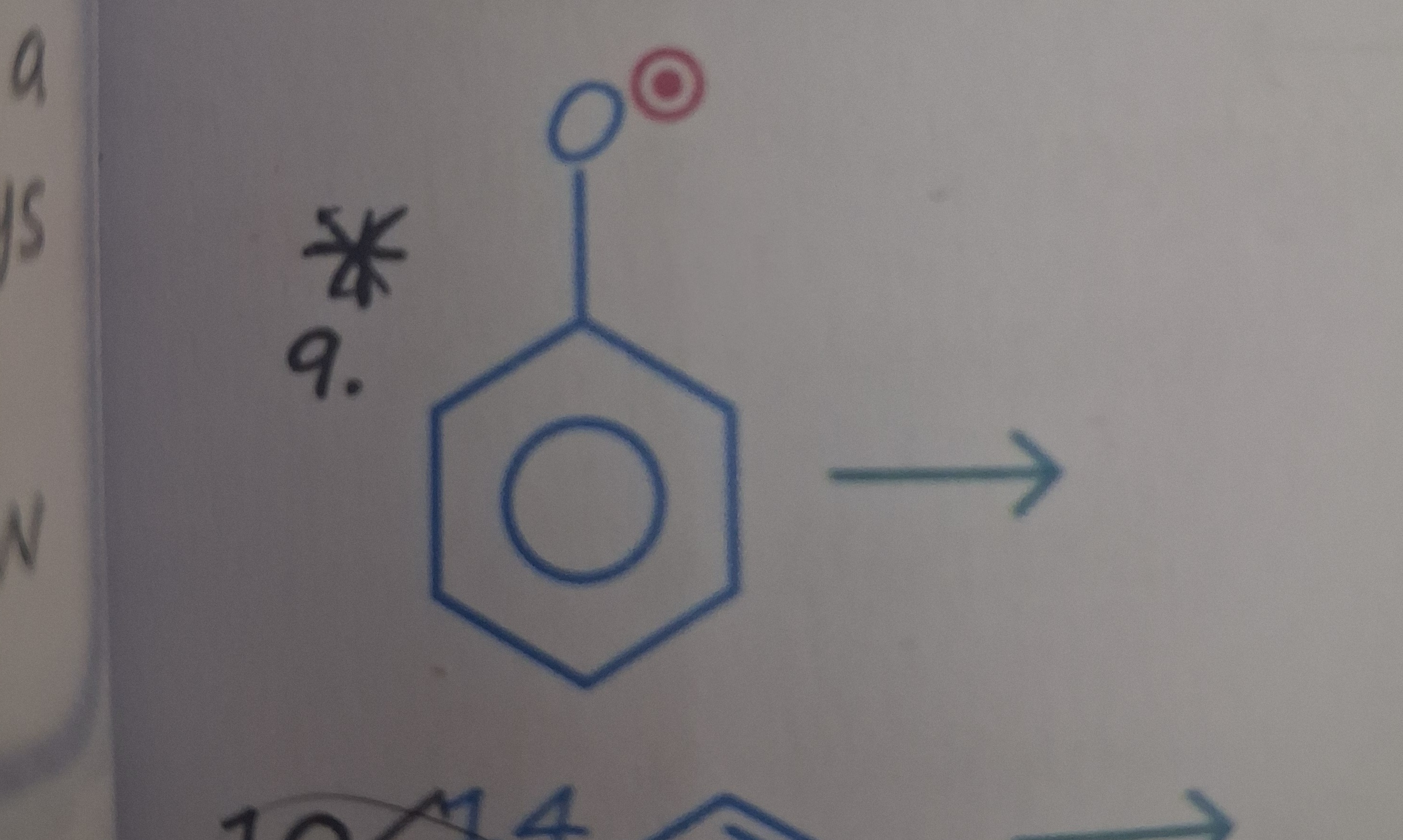
Answer
Sodium phenoxide
Explanation
Solution
The image shows phenol. The red symbol above the oxygen atom is ambiguous. However, considering the context of JEE/NEET exams and common questions about phenol, it is most likely related to its acidic nature or a reaction involving the hydroxyl group. Phenol is weakly acidic and reacts with strong bases like sodium hydroxide to form sodium phenoxide. The reaction is:
C6H5OH+NaOH⟶C6H5O−Na++H2O
If the question were asking about the reaction of phenol with a base, the product would be sodium phenoxide. If it were asking about the acidity of phenol, the answer would be that phenol is acidic.
