Question
Question: A cell is constructed as: \(Ag|AgCl_{(s)}|Cl^-(0.1M)||Pb^{2+}_{(sat.soln.ofPbCl_2)}|Pb\) Calculate...
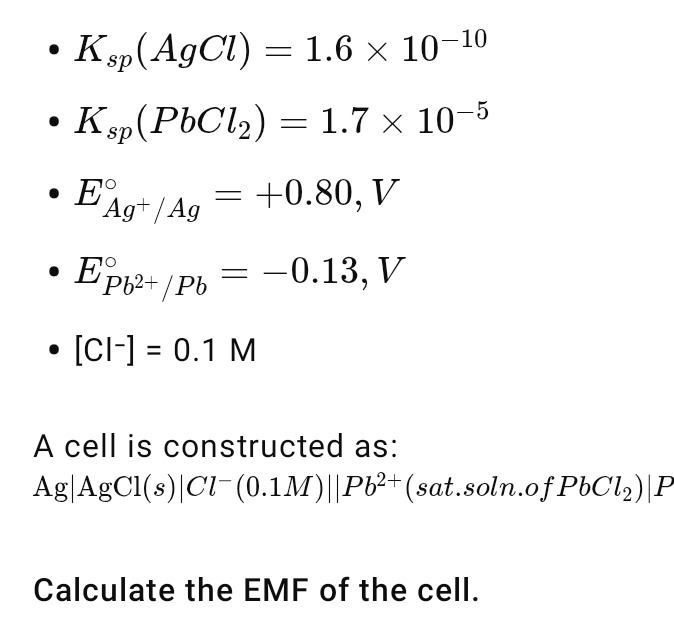
A cell is constructed as:
Ag∣AgCl(s)∣Cl−(0.1M)∣∣Pb(sat.soln.ofPbCl2)2+∣Pb
Calculate the EMF of the cell.
- Ksp(AgCl)=1.6×10−10
- Ksp(PbCl2)=1.7×10−5
- EAg+/Ag∘=+0.80,V
- EPb2+/Pb∘=−0.13,V
- [Cl−]=0.1M
-0.46 V
Solution
To calculate the EMF of the cell, we need to determine the potential of each half-cell using the Nernst equation and then apply the formula Ecell=Ecathode−Eanode.
The given cell notation is: Ag∣AgCl(s)∣Cl−(0.1M)∣∣Pb(sat.soln.ofPbCl2)2+∣Pb
1. Anode (Left Half-Cell): Ag∣AgCl(s)∣Cl−(0.1M)
The electrode reaction is AgCl(s)+e−⇌Ag(s)+Cl−.
First, we need to calculate the standard electrode potential EAgCl/Ag,Cl−∘. We know the standard potential for Ag+/Ag and the Ksp for AgCl.
The relevant reactions are:
(1) Ag(aq)++e−⇌Ag(s); E1∘=EAg+/Ag∘=+0.80V
(2) AgCl(s)⇌Ag(aq)++Cl(aq)−; Ksp=1.6×10−10
Adding reactions (1) and (2) gives the desired reaction:
AgCl(s)+e−⇌Ag(s)+Cl(aq)−
The standard free energy change for the overall reaction is the sum of the standard free energy changes for (1) and (2):
ΔGoverall∘=ΔG1∘+ΔG2∘
−nFEoverall∘=−nFE1∘−RTlnKsp
For this reaction, n=1.
−FEAgCl/Ag,Cl−∘=−FEAg+/Ag∘−RTlnKsp
Dividing by −F:
EAgCl/Ag,Cl−∘=EAg+/Ag∘+FRTlnKsp
Using FRT=0.0591 at 298 K and lnx=2.303logx:
EAgCl/Ag,Cl−∘=EAg+/Ag∘+0.0591logKsp
EAgCl/Ag,Cl−∘=0.80+0.0591log(1.6×10−10)
log(1.6×10−10)=log1.6−10=0.2041−10=−9.7959
EAgCl/Ag,Cl−∘=0.80+0.0591×(−9.7959)
EAgCl/Ag,Cl−∘=0.80−0.5790=0.2210V
Now, calculate the potential of the anode using the Nernst equation:
Eanode=EAgCl/Ag,Cl−∘−10.0591log[AgCl(s)][Ag(s)][Cl−]
Since Ag(s) and AgCl(s) are solids, their activities are 1.
Eanode=EAgCl/Ag,Cl−∘−10.0591log[Cl−]
Given [Cl−]=0.1M.
Eanode=0.2210−0.0591log(0.1)
Eanode=0.2210−0.0591×(−1)
Eanode=0.2210+0.0591=0.2801V
2. Cathode (Right Half-Cell): Pb(sat.soln.ofPbCl2)2+∣Pb
The electrode reaction is Pb(aq)2++2e−⇌Pb(s).
First, find the concentration of Pb2+ in a saturated solution of PbCl2.
PbCl_2_{(s)} \rightleftharpoons Pb^{2+}_{(aq)} + 2Cl^-_{(aq)}
Given Ksp(PbCl2)=1.7×10−5.
Let [Pb2+]=s. Then [Cl−]=2s.
Ksp=[Pb2+][Cl−]2=s(2s)2=4s3
s=(4Ksp)1/3=(41.7×10−5)1/3=(0.425×10−5)1/3=(4.25×10−6)1/3
s=(4.25)1/3×10−2≈1.6206×10−2M
So, [Pb2+]=1.6206×10−2M.
Now, calculate the potential of the cathode using the Nernst equation:
Ecathode=EPb2+/Pb∘−20.0591log[Pb2+][Pb(s)]
Since Pb(s) is a solid, its activity is 1.
Ecathode=EPb2+/Pb∘−20.0591log[Pb2+]1
Given EPb2+/Pb∘=−0.13V.
Ecathode=−0.13−20.0591log1.6206×10−21
Ecathode=−0.13−0.02955log(1.6206100)
Ecathode=−0.13−0.02955log(61.705)
log(61.705)≈1.7903
Ecathode=−0.13−0.02955×1.7903
Ecathode=−0.13−0.05289=−0.18289V
3. Calculate the EMF of the Cell:
Ecell=Ecathode−Eanode
Ecell=−0.18289−0.2801
Ecell=−0.46299V
Rounded to two decimal places, Ecell=−0.46V.
The negative EMF indicates that the reaction as written (oxidation at Ag electrode and reduction at Pb electrode) is non-spontaneous. The spontaneous reaction would be the reverse.
Explanation of the solution:
-
Calculate Standard Potential for AgCl/Ag, Cl- Electrode:
The standard potential for the reaction AgCl(s)+e−⇌Ag(s)+Cl− is derived from EAg+/Ag∘ and Ksp(AgCl) using the relationship:
EAgCl/Ag,Cl−∘=EAg+/Ag∘+0.0591logKsp(AgCl)
EAgCl/Ag,Cl−∘=0.80+0.0591log(1.6×10−10)=0.80+0.0591×(−9.7959)=0.2210V
-
Calculate Potential of Anode (Ag|AgCl, Cl-) Electrode:
Using the Nernst equation for the reduction reaction AgCl(s)+e−⇌Ag(s)+Cl−:
Eanode=EAgCl/Ag,Cl−∘−0.0591log[Cl−]
Given [Cl−]=0.1M:
Eanode=0.2210−0.0591log(0.1)=0.2210−0.0591×(−1)=0.2210+0.0591=0.2801V
-
Calculate Concentration of Pb2+ in Cathode (Pb2+|Pb) Electrode:
The cathode involves a saturated solution of PbCl2.
For PbCl_2_{(s)} \rightleftharpoons Pb^{2+} + 2Cl^-, Ksp=[Pb2+][Cl−]2.
Let [Pb2+]=s, then [Cl−]=2s.
Ksp=s(2s)2=4s3
s=(4Ksp)1/3=(41.7×10−5)1/3=(4.25×10−6)1/3≈1.6206×10−2M
So, [Pb2+]=1.6206×10−2M.
-
Calculate Potential of Cathode (Pb2+|Pb) Electrode:
Using the Nernst equation for the reduction reaction Pb2++2e−⇌Pb(s):
Ecathode=EPb2+/Pb∘−20.0591log[Pb2+]1
Given EPb2+/Pb∘=−0.13V:
Ecathode=−0.13−20.0591log1.6206×10−21
Ecathode=−0.13−0.02955log(61.705)=−0.13−0.02955×1.7903=−0.13−0.05289=−0.18289V
-
Calculate EMF of the Cell:
Ecell=Ecathode−Eanode
Ecell=−0.18289V−0.2801V=−0.46299V
Rounded to two decimal places, Ecell=−0.46V.
